You're Almost There...

MedTech is complex. Your QMS shouldn’t be.
Greenlight Guru’s eQMS is purpose-built to help medical device companies develop and manage devices throughout the entire product lifecycle.
Get A DemoDo you know what your current system is really costing you?
According to the 2025 Greenlight Guru Benchmark Report, 34% of post-market teams uncovered critical process gaps during audits, revealing issues they hadn’t even known existed. Greenlight Guru was built to fix that.
Scale without roadblocks
Compliance you can count on
Onboard fast, prove value faster
Launch with confidence
Unite your product and quality teams
For Quality Teams
Learn MoreFor Product Development Teams
Learn MoreThe smarter way to scale your quality system
A single system for the entire
device lifecycle
Whether you’re bringing a device to market for the first time or managing a portfolio of products, you need a QMS that keeps you organized and compliant at every step.
Design controls that build your DHF for you
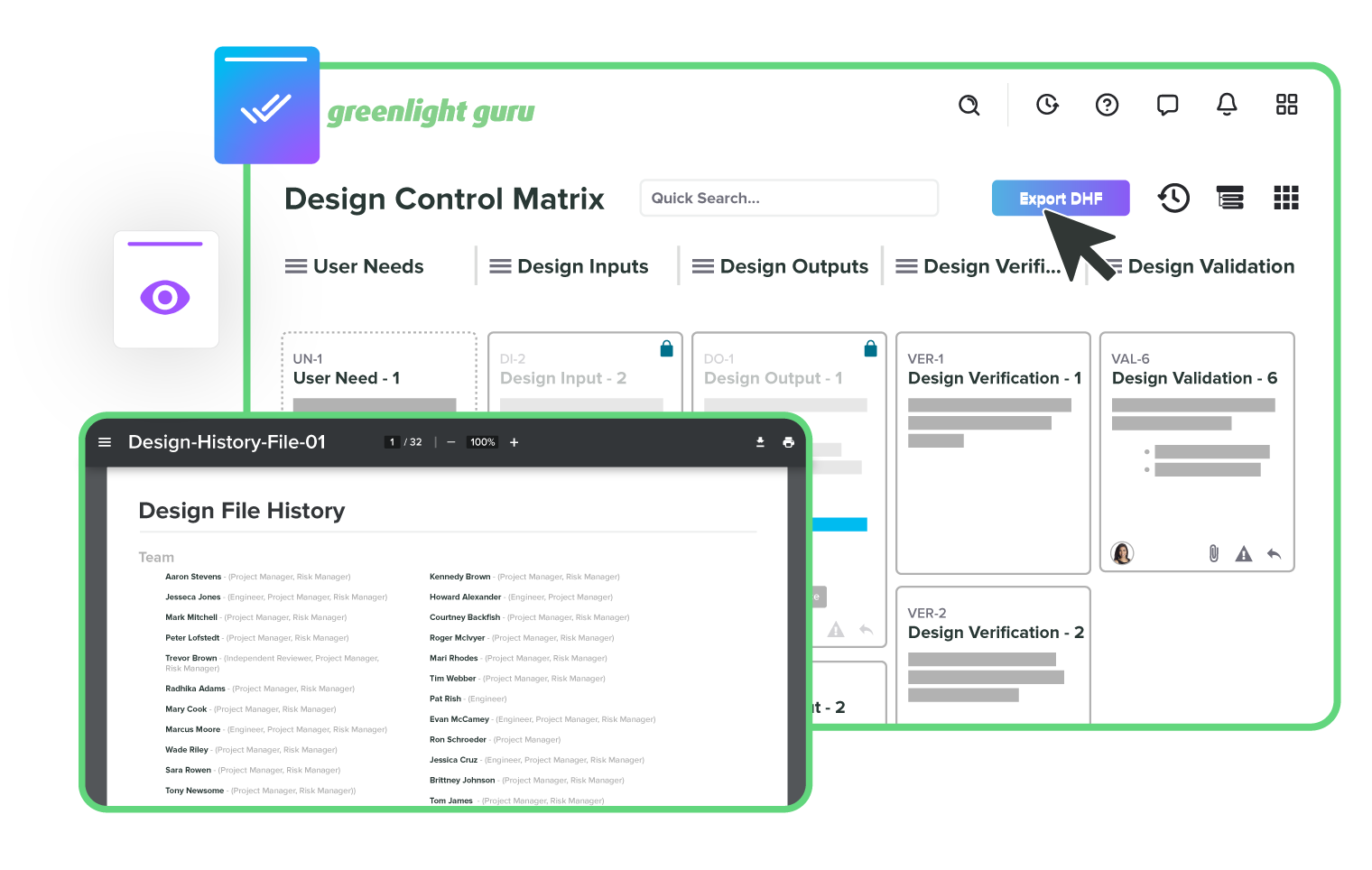
All your documents, all in one place
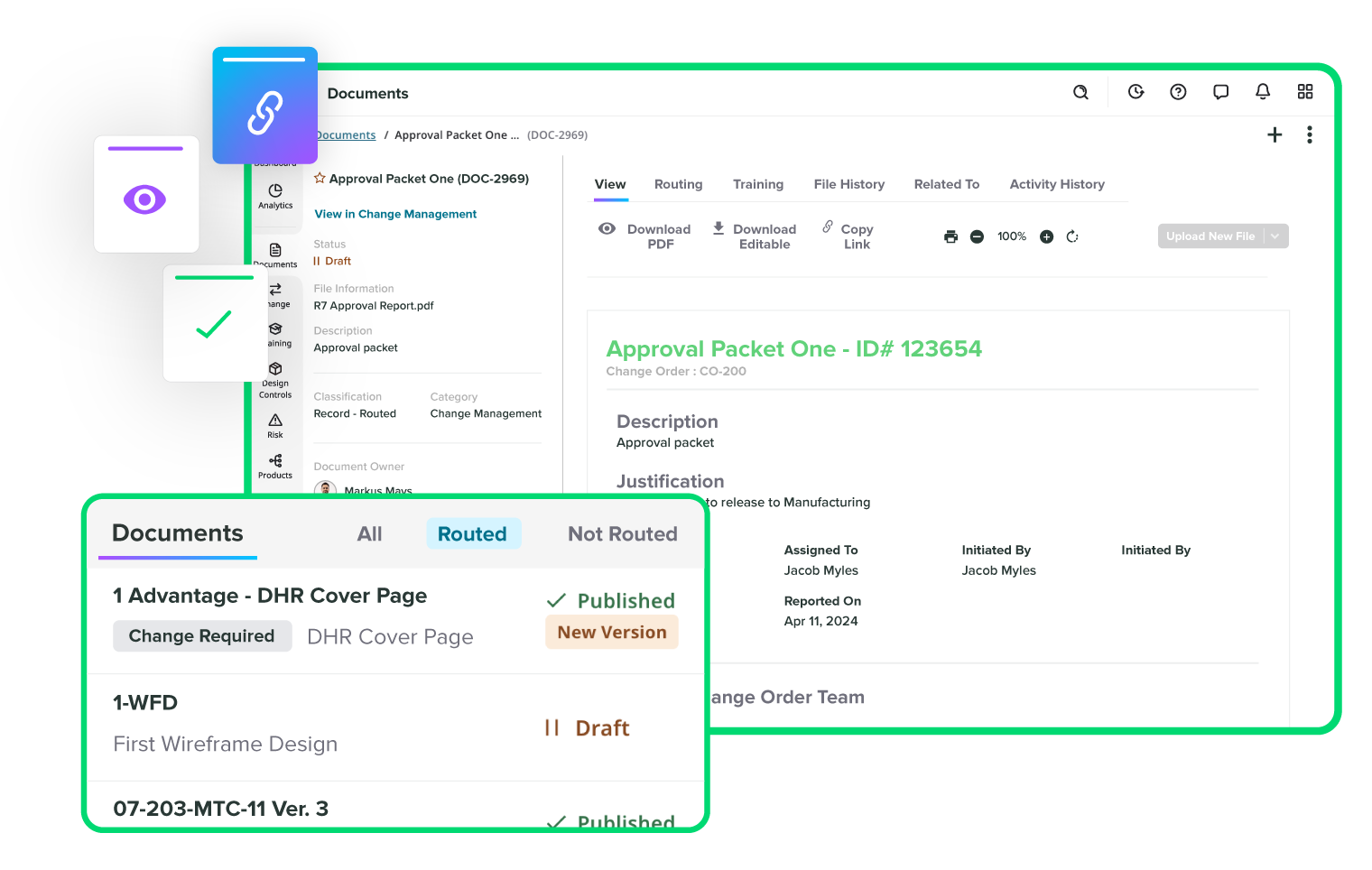
Track every change without missing a step
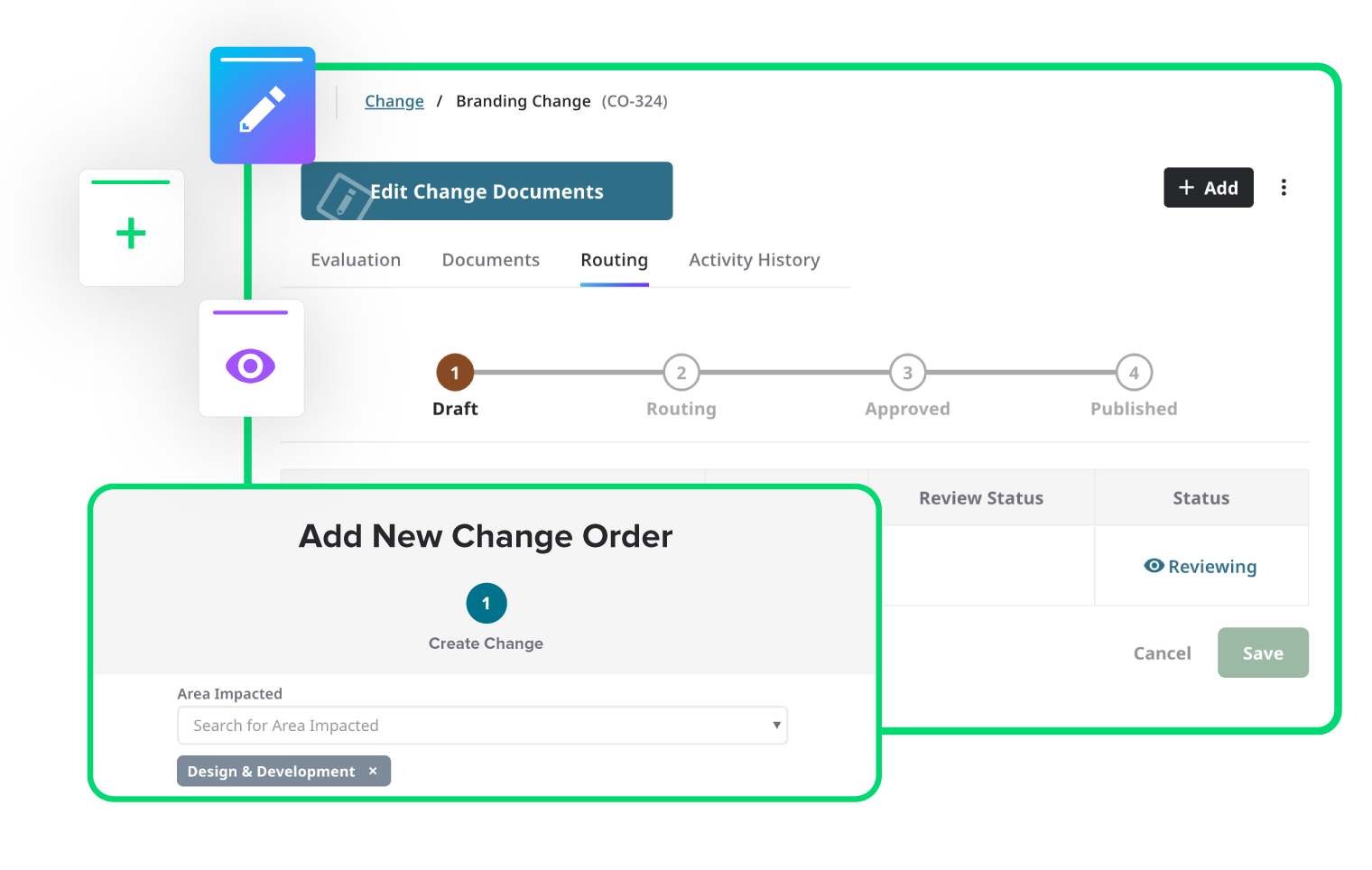
Integrate risk throughout the product lifecycle
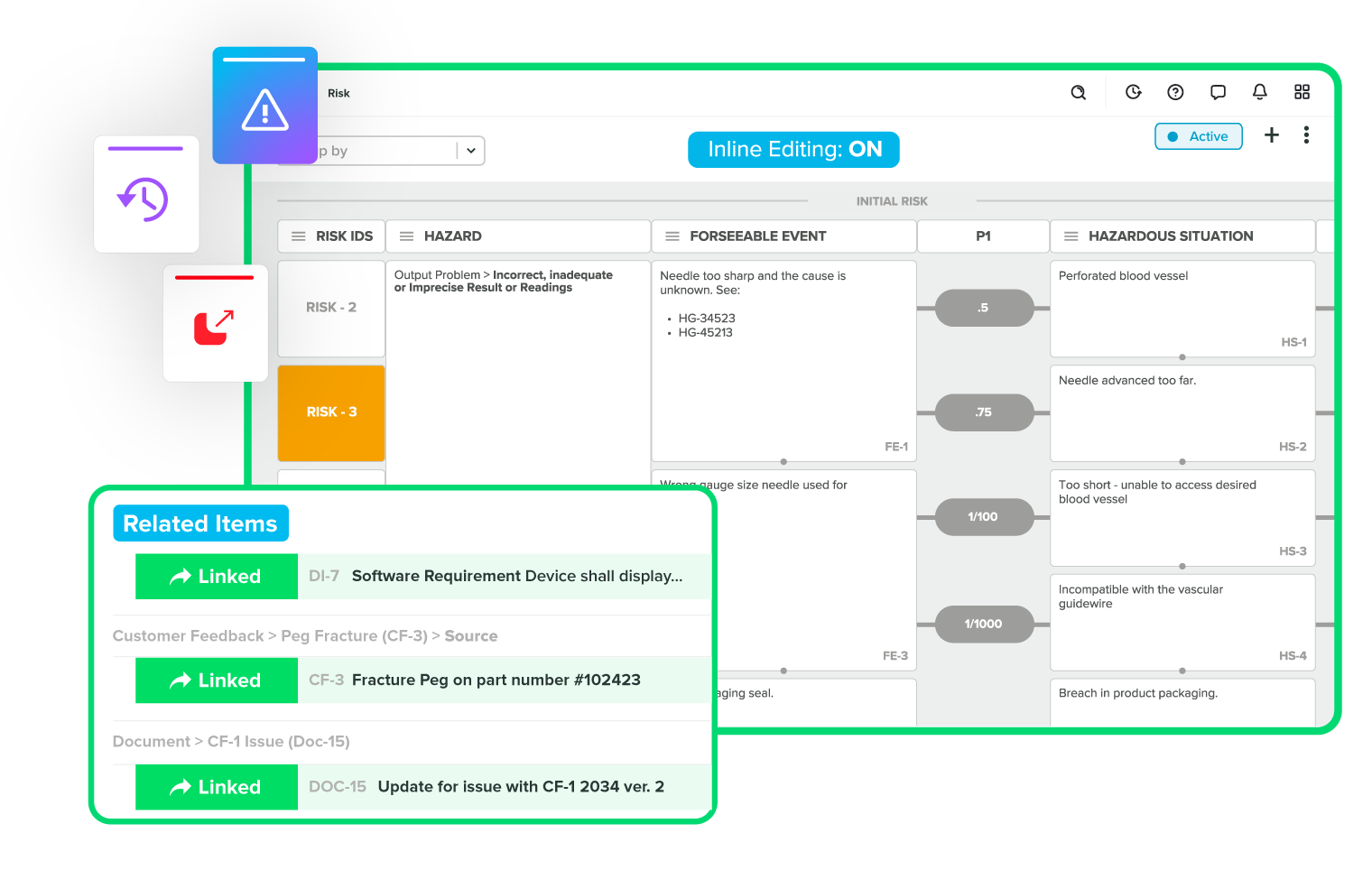
Resolve issues quickly with full traceability
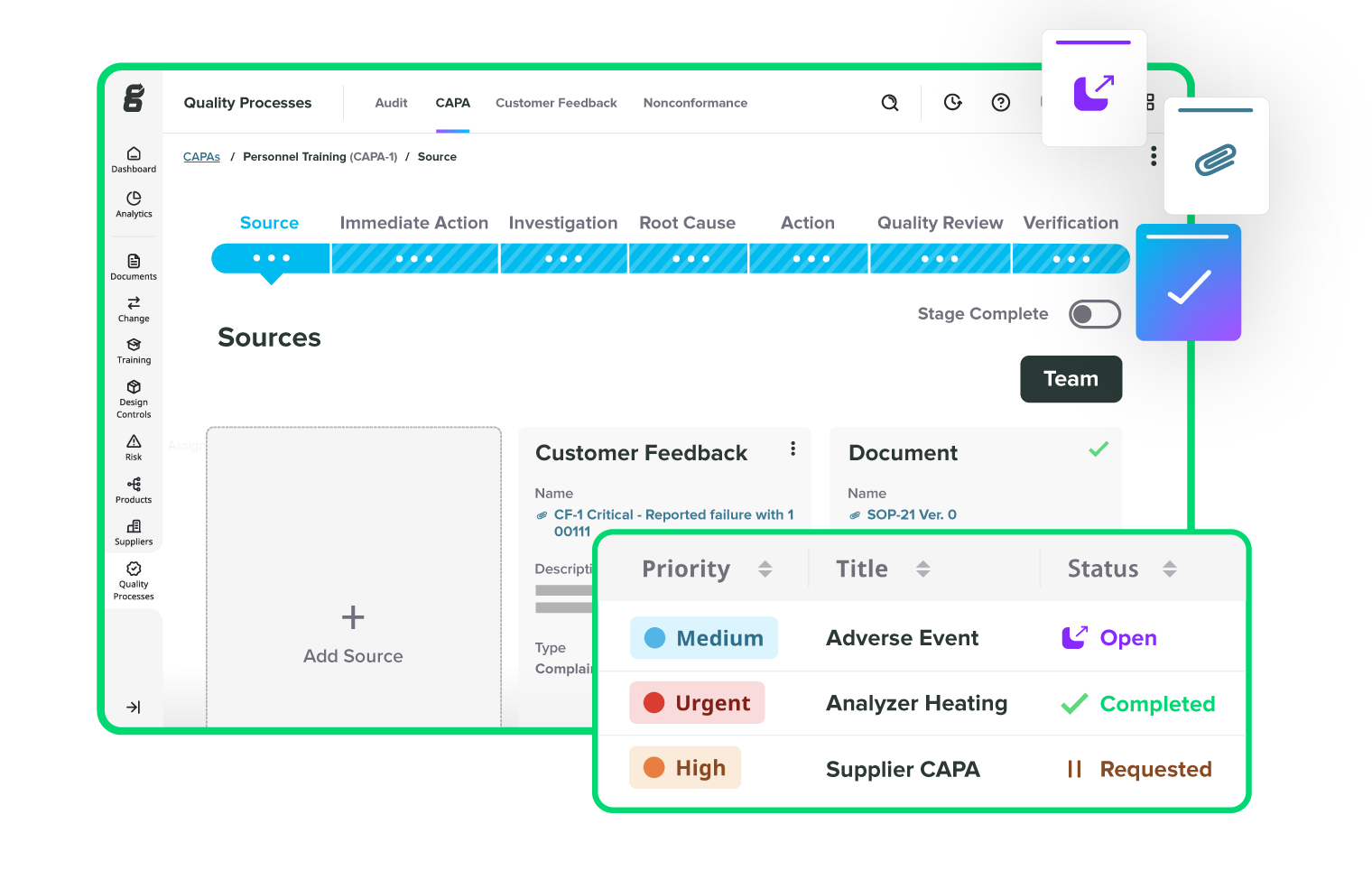
Take the pain out of audits
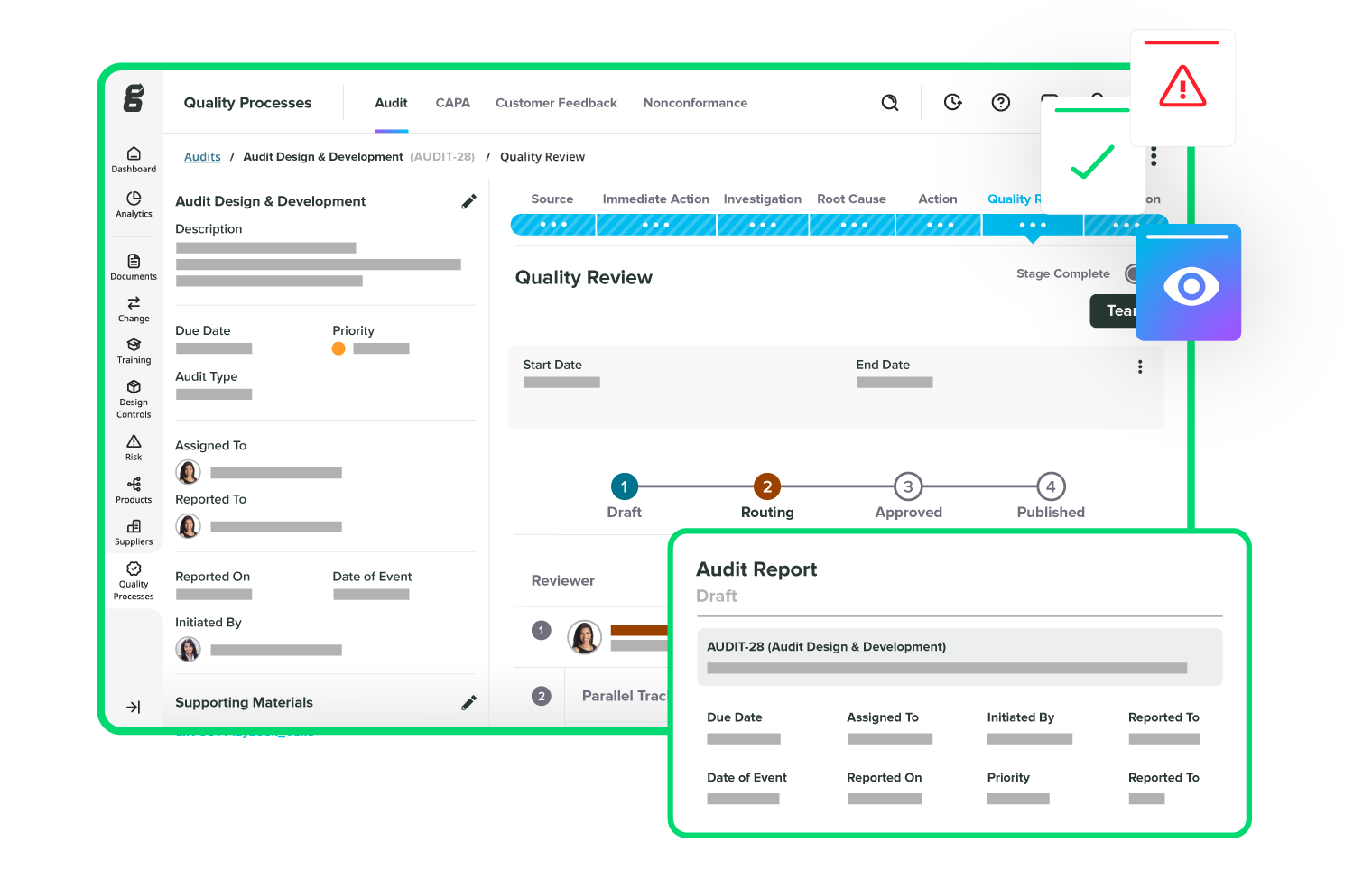
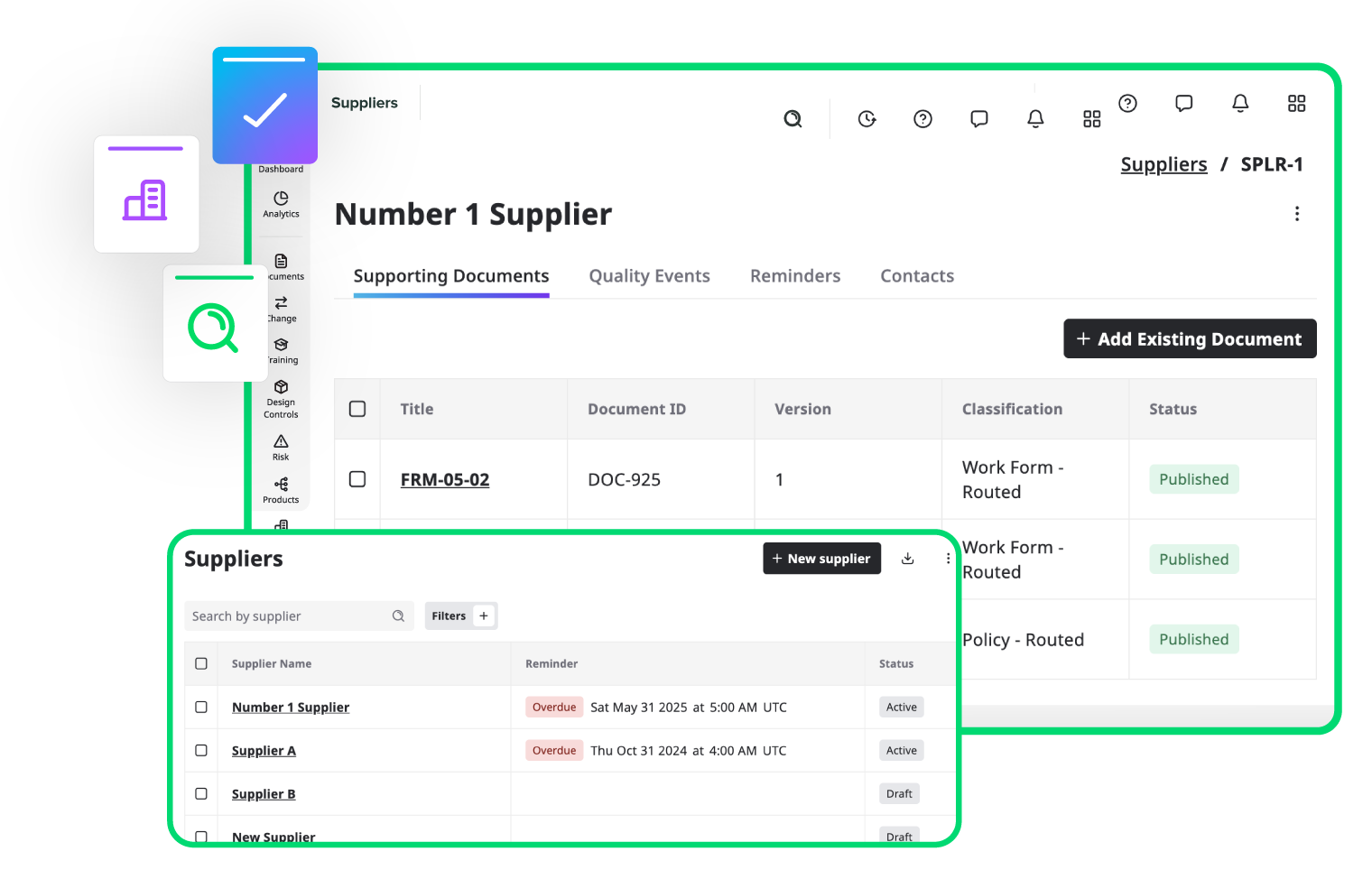
Track and manage all your suppliers
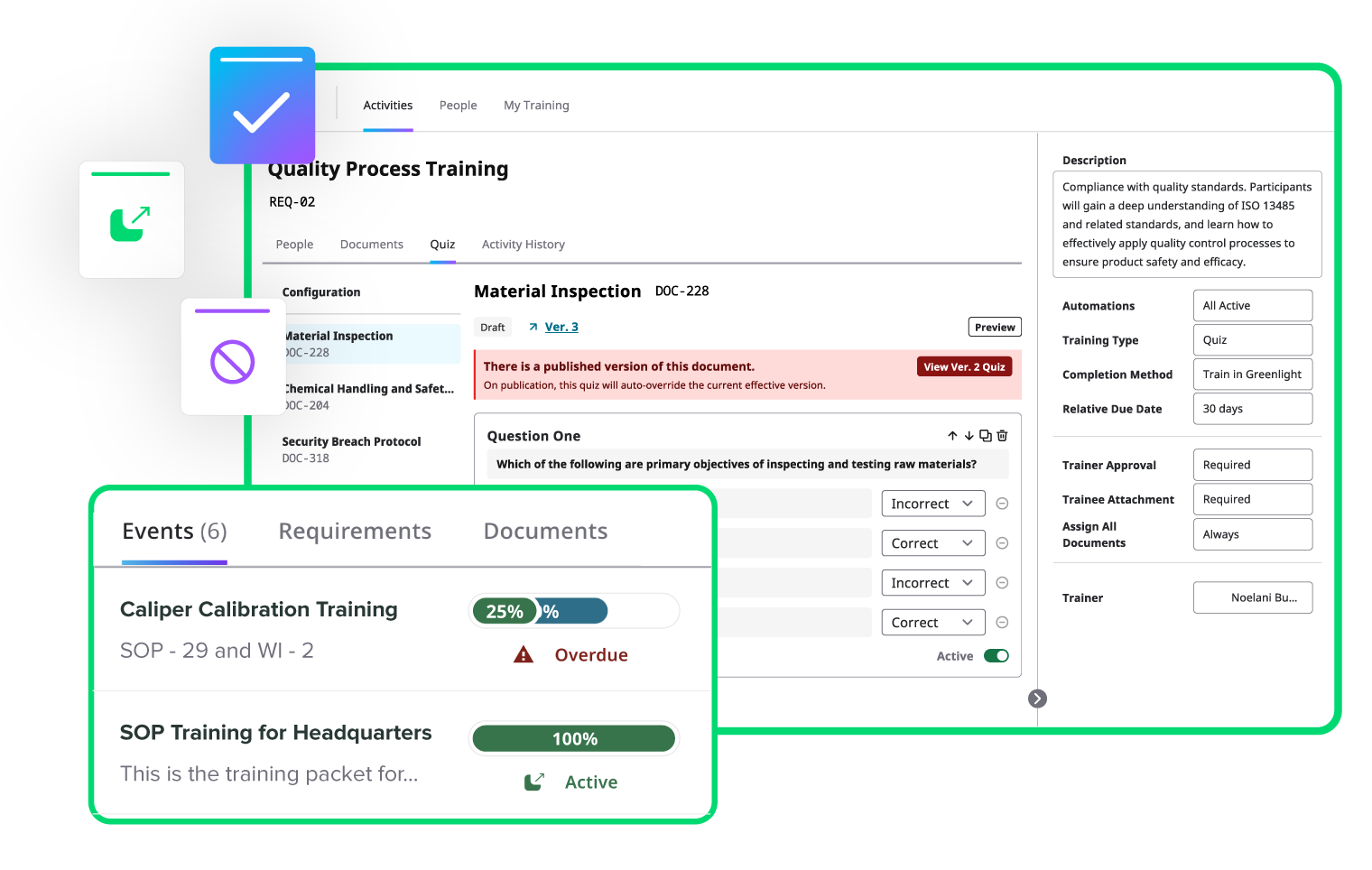
Automate training to keep your team compliant
The smarter way to scale your quality system
A single system for the entire
device lifecycle
Whether you’re bringing a device to market for the first time or managing a portfolio of products, you need a QMS that keeps you organized and compliant at every step.
Be confident in your compliance.
 +
+
-1.png)
— Clive Seymour, CEO
The problems are complicated. The solution is simple.
Boost collaboration and eliminate preventable delays with a validated QMS solution that’s built specifically for medical device companies.
Always audit-ready
Maintain complete traceability and version control in one system and stay ready for any inspection, any time.
Efficient compliance
Automate quality tasks like training, changes, and CAPAs so you can stay compliant with less manual work.
Accelerate development
Speed up product development with built-in design controls and risk management that improve team collaboration.
Growth on your terms
Support new teams, products, and markets with a QMS that scales as fast as you do.
Frequently asked questions
What is the implementation process for Greenlight Guru?
How do I justify the cost of QMS software to leadership or the board?
Do I need a QMS if we haven’t launched our product yet?
Why can’t we keep doing this on Excel?
Will Greenlight Guru work for my type of device or business model?
What if our team’s workflow doesn’t match yours?
Why should we choose a MedTech-specific solution over a larger provider that serves multiple industries?
What makes GG different from just “another tool” that no one uses?
Will Greenlight Guru integrate with our existing tools (e.g., ERP, Jira, etc.)?