Greenlight Guru Clinical enables collection of a wide range of patient-specific data, including outcomes, usability feedback, clinical experience, safety and vigilance events, and post-market survey or registry data. The Cases module is designed for ad-hoc, prospective data capture in post-market settings.
Studies can be built in minutes with Greenlight Guru Clinical’s intuitive 3-step builder. The Cases module allows teams to launch studies in as little as 90 seconds, eliminating delays and accelerating data collection.
Yes. While the Cases module focuses on post-market data, Greenlight Guru Clinical supports both pre-market and post-market evidence needs, including registries, pivotal trials, PMA studies, and PMCF surveys.
Yes. Teams can adapt and reuse existing forms and templates across multiple studies or device families, ensuring consistency and reducing the need to start from scratch for each project.
Unlike traditional EDC or CTMS tools which often require rigid study structures and lengthy setup, the Greenlight Guru Clinical Cases module is optimized for flexible, ad hoc data collection in post-market environments. It supports decentralized workflows, rapid setup, and subject-specific form delivery, making it ideal for PMCF, registry, or complaint-related studies where pre-scheduled visits aren't feasible.
Yes. The Cases module is designed for use cases that do not require fixed visits. Forms can be filled out independently by investigators, sites, or subjects whether for a one-time event like a complaint or recurring follow-ups in a registry.
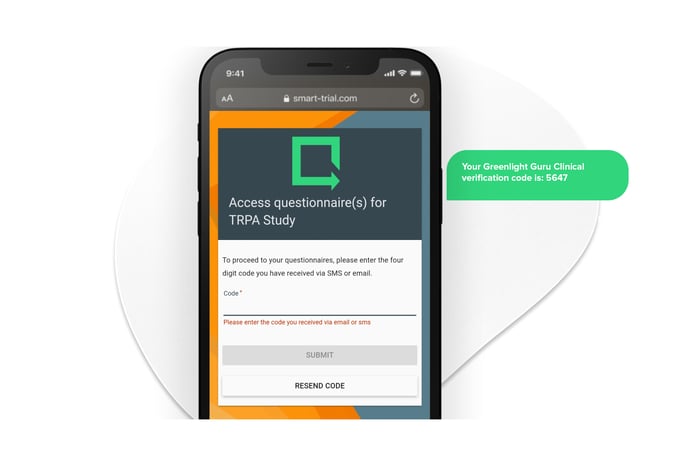
The Cases module supports diverse user roles including sponsors, monitors, investigators, and even subjects via ePRO or eConsent. Study owners can control access with role-based permissions and ensure that each stakeholder sees only what is relevant to them.
Yes. Greenlight Guru Clinical including the Cases module is validated and aligned with ISO 14155:2020, FDA 21 CFR Part 11, ICH-GCP, GDPR, and HIPAA. The system features electronic signatures, audit trails, and full traceability to support inspection readiness.
Absolutely. Each case in the module can be tied to individual subject IDs, device details, event types (such as adverse events or complaints), and more, providing structured, filterable insights across your dataset.
Monitors can review data using a dedicated read-only interface, raise queries, and lock form answers once verified. The built-in audit log captures all activity to ensure transparency and compliance.