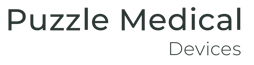Solutions built for medical device teams to move faster, stay compliant, and grow with confidence.
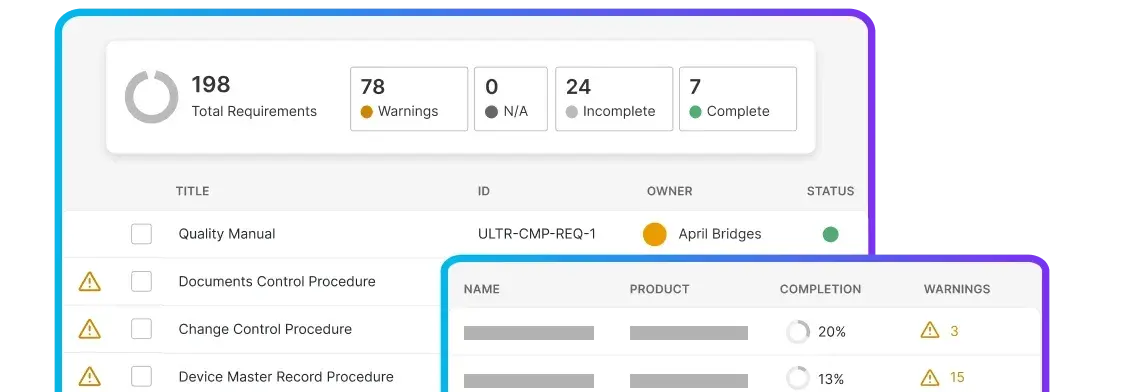
Manage quality events, training, and documentation in one place.
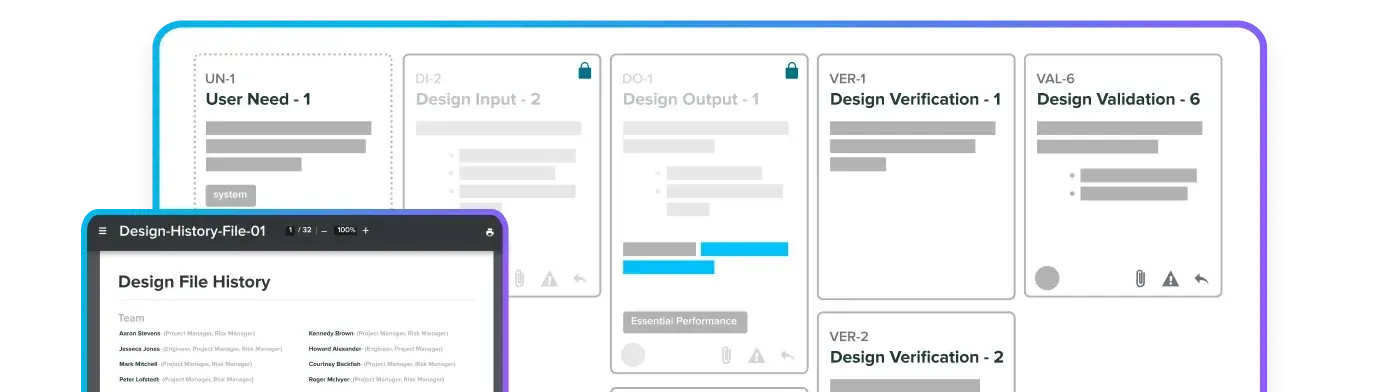
Control documents, training, and change with traceability.
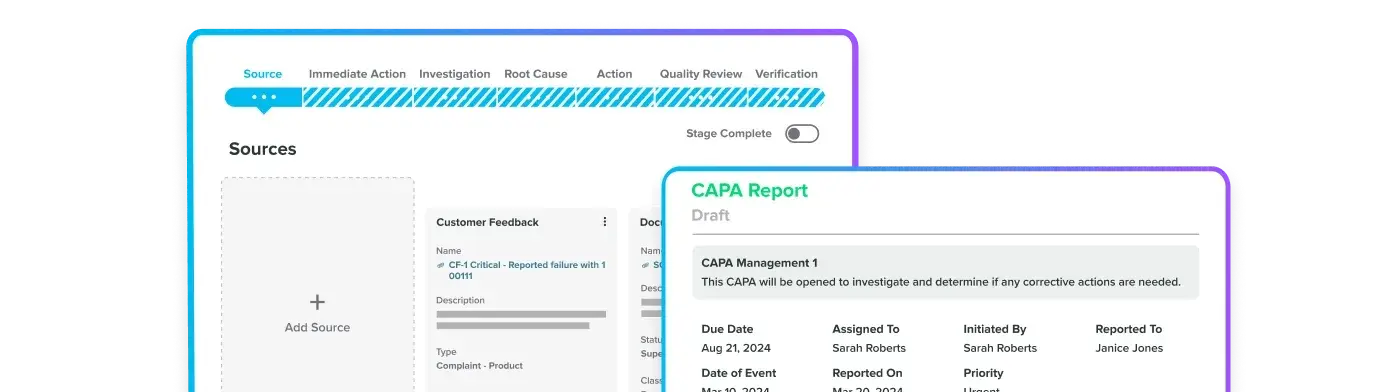
Track CAPAs, audits, and NCs with audit-ready records.
Qualify suppliers and manage parts with visibility.
Connect design, risk, and AI-powered traceability as you build.
Link needs, requirements, and verification to design controls.
Automate traceability from dev tools for faster releases.
Assess ISO 14971 risk as requirements and tests evolve.
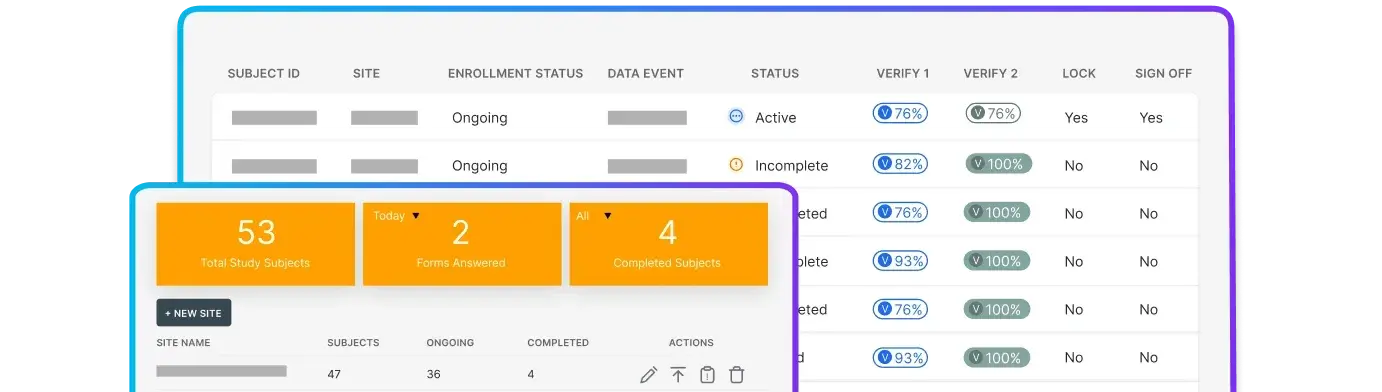
Oversee studies with connected data, documents, and workflows.
Capture compliant clinical data with a validated medical EDC.
Plan and manage PMCF aligned to EU MDR expectations.
Collect clinical outcome data in-person or remotely on any device.
Built with regulatory insight to help medtech teams move faster with confidence.
Find issues faster and make smarter decisions with less manual work.
Tools and resources that help teams implement quickly and meet requirements.