Introducing Risk Reviews for Efficient, Compliant Risk Management

This week at Greenlight Guru, we released several new updates to Greenlight Guru Quality, including Risk Reviews, the latest addition to our new Risk Management workspace.
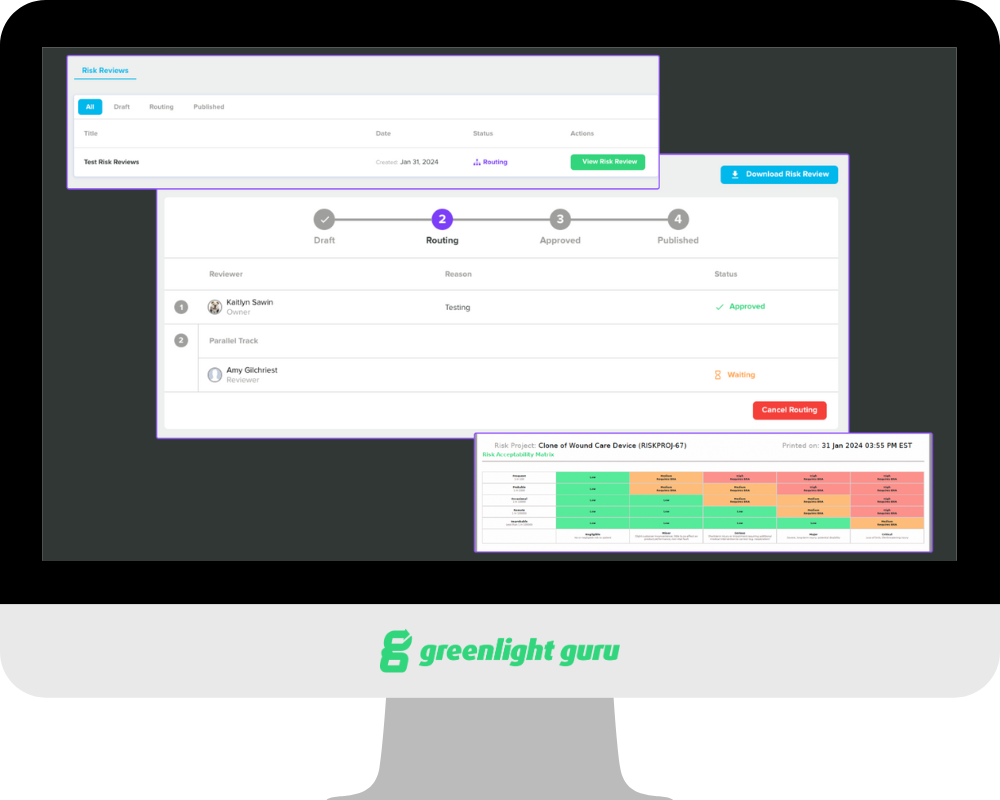
With Risk Reviews, our customers now have a dedicated workflow for product and engineering teams to review and approve their risk matrix. While this capability was previously combined with our design review functionality, separating Risk Reviews helps drive the risk management process independently, ensuring compliance and fostering collaboration.
The result is a purpose-built risk workflow that helps your team enhance product safety, accelerate time-to-market while streamlining post-market activities, and build a robust, audit-ready risk management file (RMF) with confidence.
BONUS RESOURCE: Click here to download a free, customizable Risk Management Plan Template.
What are risk reviews and why are they so important?
Risk reviews (or risk management reviews, as they’re also known) are an integral step in the risk management process laid out in ISO 14971:2019, and an essential part of your RMF.
As MedTech companies progress through the risk management process in ISO 14971:2019, they’re required to document all their activities, and those documents and records should be stored in the RMF and available to auditors. Those documented activities include your risk management plan, risk assessment (analysis plus evaluation), risk controls, and overall residual risk acceptability.
Before your device can be placed on the market, however, you are required to review the records and documentation of all those activities in the risk management file. As ISO 14971:2019 states in Section 9:
Prior to release for commercial distribution of the medical device, the manufacturer shall review the execution of the risk management plan. This review shall at least ensure that:
-
-
the risk management plan has been appropriately implemented;
-
-
- the overall residual risk is acceptable; and
-
-
appropriate methods are in place to collect and review information in the production and post-production phases.
-
The record of your completed risk review, known as the risk management report, will also become part of your risk management file.
What does Risk Reviews allow Greenlight Guru customers to do?
Risk Reviews gives users a way to generate an artifact that can be routed for review by the product development team. This document includes the risk acceptability matrix, risk matrix, and lists of all hazards, foreseeable events, hazardous situations, and harms for the device.
Risk Reviews also allows users to lock the risk project during review to ensure there are no changes to the project that might affect its outcome or render it obsolete by the time the review is complete.
The document that Risk Reviews generates, along with the outcome of the risk review, can then be added to your risk management file with the rest of your records and documentation related to risk.
Both Quality and Product Development teams will find Risk Reviews to be an efficient and simple way to perform risk management reviews, stay compliant with regulations, and build an audit-ready risk management file.
BONUS RESOURCE: Click here to download a free, customizable Risk Management Plan Template.
Continued product enhancements in the MedTech Suite
Risk Reviews gets the spotlight today, but we’re continuing to add new product features and enhancements to our MedTech Suite of purpose-built solutions.
In the Training Management workspace, our team is enhancing the workflows for training management to make them complete, comprehensive, and automated. This will allow teams to streamline their training compliance and keep the right people trained on the right information at the right time.
We’re also working to help MedTech companies bridge the gap between critical business systems with APIs that reduce manual data handling, eliminate data silos, and help teams scale quality data across their business.
If you’re ready to see the latest updates to Greenlight Guru Quality, get your free, personalized demo of Greenlight Guru today →
Tory Lopez is a Senior Manager of Content Marketing at Greenlight Guru, the #1 provider of purpose-built MedTech solutions, with 5+ years of experience in the medical device industry and 10+ years in sales and marketing. Tory leads a dynamic team of writing and creative professionals, overseeing content strategy,...
Related Posts
[LIVE] Design Controls, Development, and Risk for Software as a Medical Device (SaMD)
Introducing Risk Solutions: A New Era in Risk Management for MedTech
Greenlight Guru Updates Risk Management Capabilities to Align with ISO 14971:2019, Further Enhances Change Management Capabilities
Get your free download
Risk Management Plan Template