Definition
A risk matrix is a tool used by medical device engineers to quantify the risk level associated with a medical device. Risk matrices are useful because they offer standardized criteria and consistent methodology for assessing the risk of medical devices and classifying them appropriately.
Whether your medical device company intends to sell devices in Europe, Canada, the United States, or all of the above, risk assessment and management will play an important role in your product development activities and the overall planning of your quality management activities and to-market strategy.
A risk matrix is a tool that medical device designers and engineers can use to assess the risk associated with a specific type of incident. Risk matrices are used in conjunction with other tools like benefit-risk analysis to quantify risk, map potential sources of harm to corresponding design features, and make decisions about how to reduce the risk of a device. The international standard for risk management in medical device manufacturing is known as ISO 14971.
FREE DOWNLOAD: Click here to download our Risk Management Plan Template for medical device companies.
Medical Device Engineers Use Benefit-Risk Analysis To Quantify Risk
When medical device engineers design a new medical device, they perform a benefit-risk analysis. A benefit-risk analysis takes the probability of occurrence of HARM and the consequences of that harm and justifies those based on the overall benefit of the medical device for the end user. Risk can be quantified with the following formula:
Risk = Severity of Harm (S) x Probability of Occurrence of Harm (POH).
(We explain this in depth here, using the grossest example ever!)
Each hazard is assigned a score between 1 and 5 for its severity (consequences) and probability (likelihood that the failure mode would occur). The product of these two scores is used as a relative measure of risk for that particular hazard.
Building The Risk Assessment Matrix: Risk Likelihood And Consequences
A risk matrix is used to assign a relative descriptor of "high risk," "medium risk" or "low risk" to a specific failure mode depending on its likelihood and consequences. Once engineers have assessed the likelihood of a particular failure mode occurring and worked to understand the potential consequences of such a failure, a risk matrix can be used to quantify the risk and determine whether it is justified by the benefits of the device.
Risk matrices are typically organized with "Failure Mode Likelihood" on the Y-axis and "Failure Mode Consequences" on the X-axis. Failure modes are given a rating between 1 to 5 based on the following scale:
- Rare - The failure mode is not expected to ever happen, but it is still within the realm of possibility.
- Unlikely - The failure mode could occur occasionally, but is not expected to occur frequently.
- Likely - The failure mode could occur in many circumstances.
- Expected - The failure mode is expected to occur in most circumstances.
- Certain - The failure mode will occur each time the product is used.
In addition, the following five-point scale is used to measure the consequences of a specific failure mode:
- Minor - The failure mode leads to a minor injury or adverse health outcome.
- Moderate - The failure mode results in a moderate injury or adverse health outcome.
- Serious - The failure mode results in a major injury or adverse health outcome.
- Major - The failure mode can result in the death of the patient.
- Catastrophic - The failure mode is likely to lead to multiple deaths.
Engineers can obtain a risk score by multiplying together the scores for failure modes and consequences for a given failure mode. As a rule of thumb, a risk score between 1 and 4 is considered low, while a score between 5 and 8 is medium risk and anything over 8 is considered high risk.
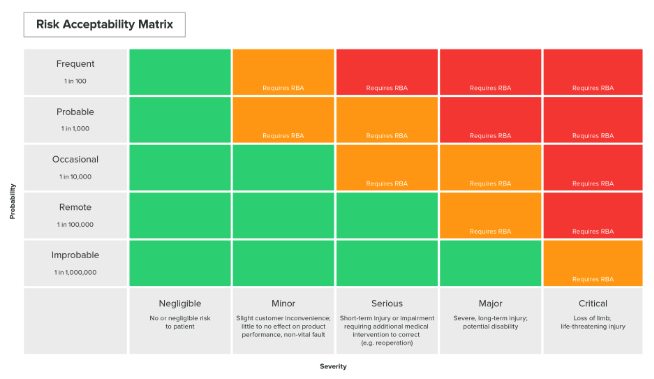
(A Risk Matrix example in the Greenlight Guru Software. Click here to learn more about our Risk Management Software.)
Greenlight Guru Helps Maintain Accurate Records of Risk Management Activities
Medical device manufacturers are held accountable for the safety and effectiveness of their medical devices by regulatory bodies everywhere that medical devices are sold. If your medical device company is audited by the FDA, you will need to produce documented evidence of your risk management activities, including the use of a risk assessment matrix to analyze the risk of your medical device.
Greenlight Guru offers the only software-based QMS designed exclusively for medical device companies. With Greenlight Guru's integrated interfaces for design controls and risk management, medical device companies can easily track adverse events, identify sources of harm related to specific design outputs and make better decisions about how to manage and reduce risk.
FREE DOWNLOAD: Click here to download our Risk Management Plan Template for medical device companies.
Related Links








