You're Almost There...

The only eClinical platform for medical devices
Greenlight Guru Clinical is built to help medical device companies generate high-quality, reliable, and compliant clinical data from every study.
Get A DemoBad data quality can easily ruin a perfectly good study
Data collected on paper or spreadsheets is guaranteed to have errors and may be unusable in submissions. Greenlight Guru eliminates errors for high-quality data from every source.
Built for MedTech
Fast implementation
Unrivaled visibility
Any study, anywhere
Collect data in-house or for clients
For Clinical Teams
Learn MoreFor CROs
Learn MoreCollect and manage all your clinical data
A single, validated platform with everything you need to run medical device studies
Medical device trials are unique. Greenlight Guru is built to help MedTech companies carry out GCP-compliant studies that generate high-quality clinical data.
Unrivaled data visibility and management
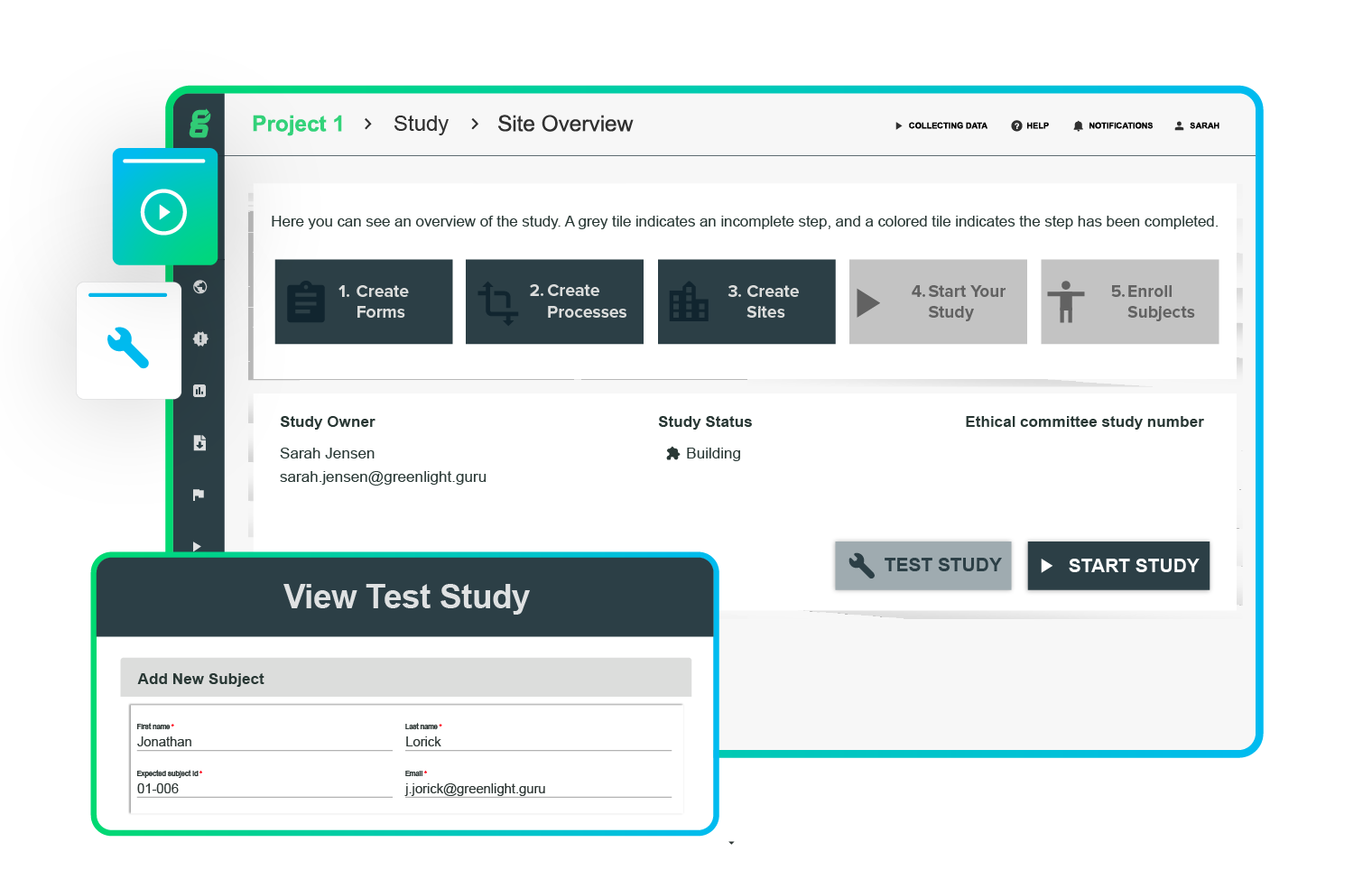
Get higher response rates with patient-friendly ePRO
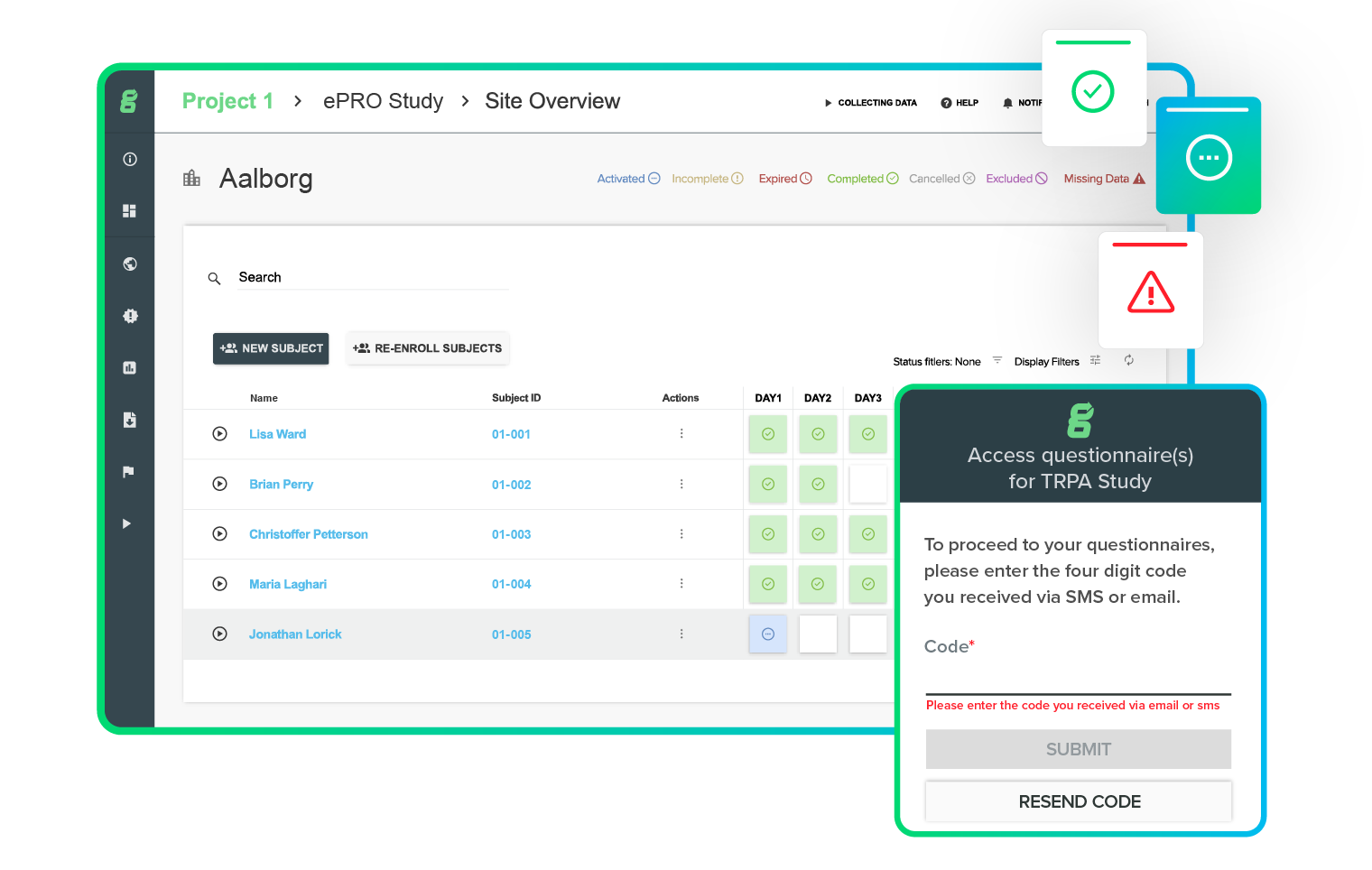
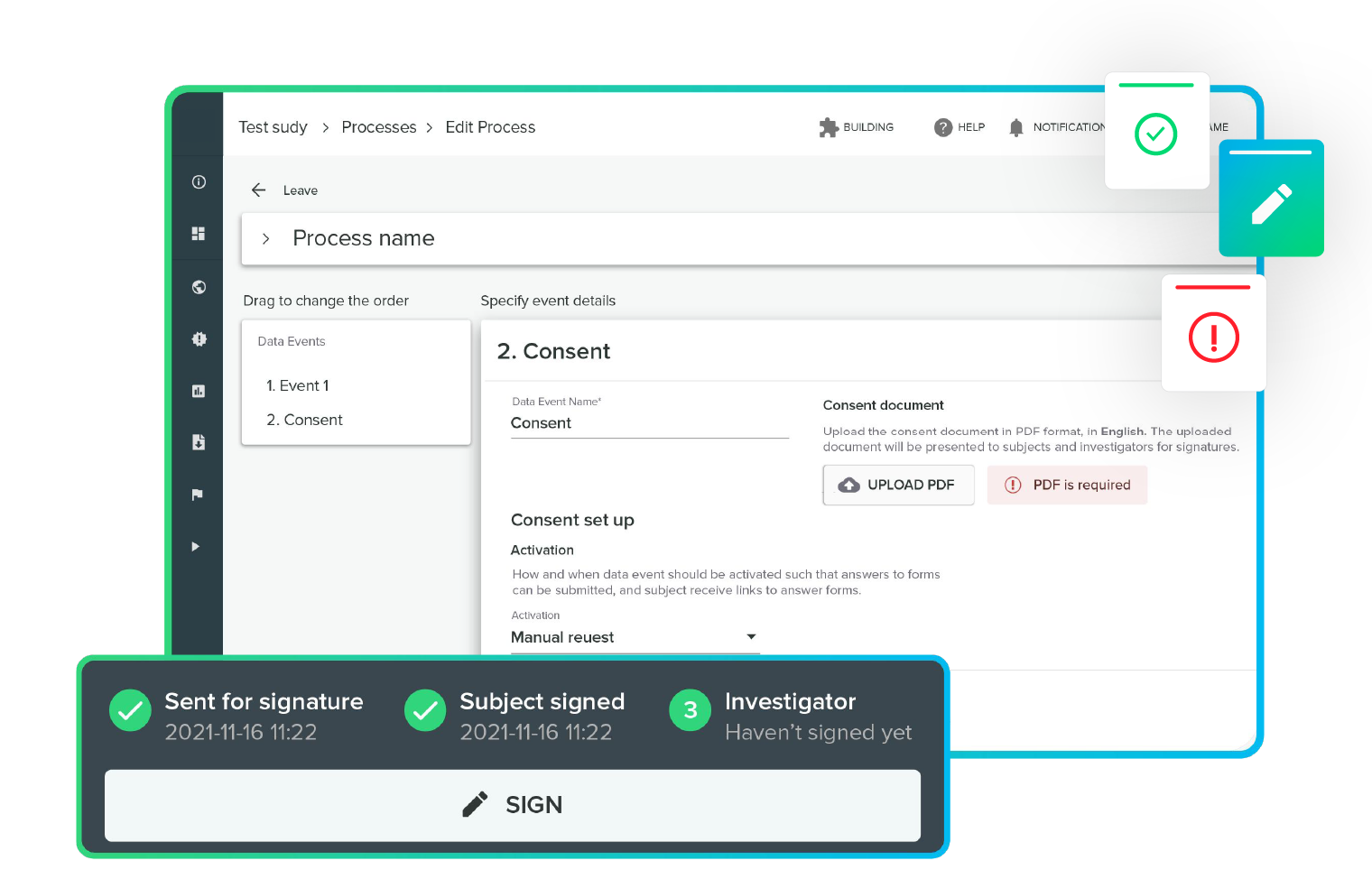
Fast and friendly informed consent
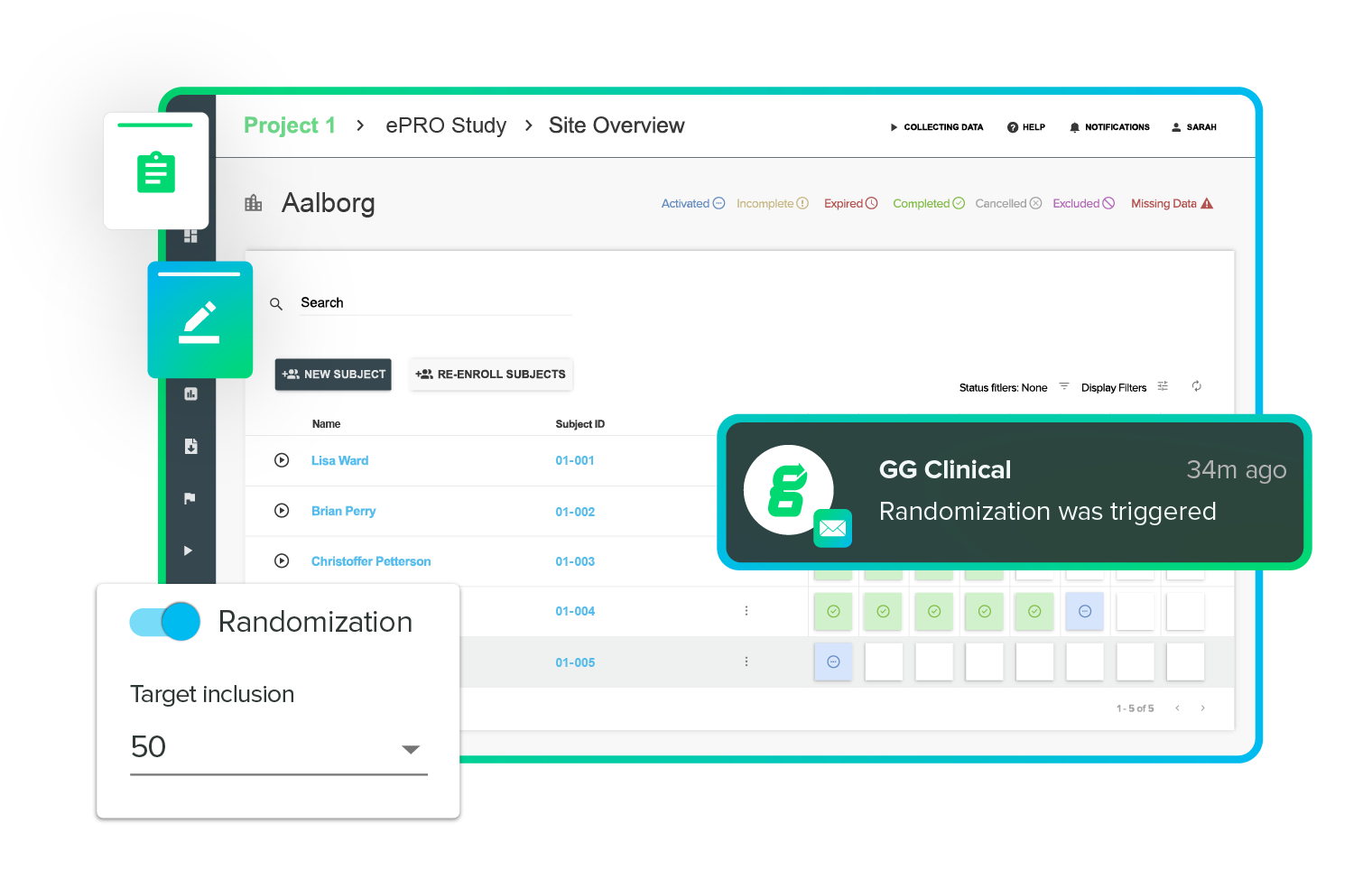
Add randomization to any study
Collect post-market data from any source
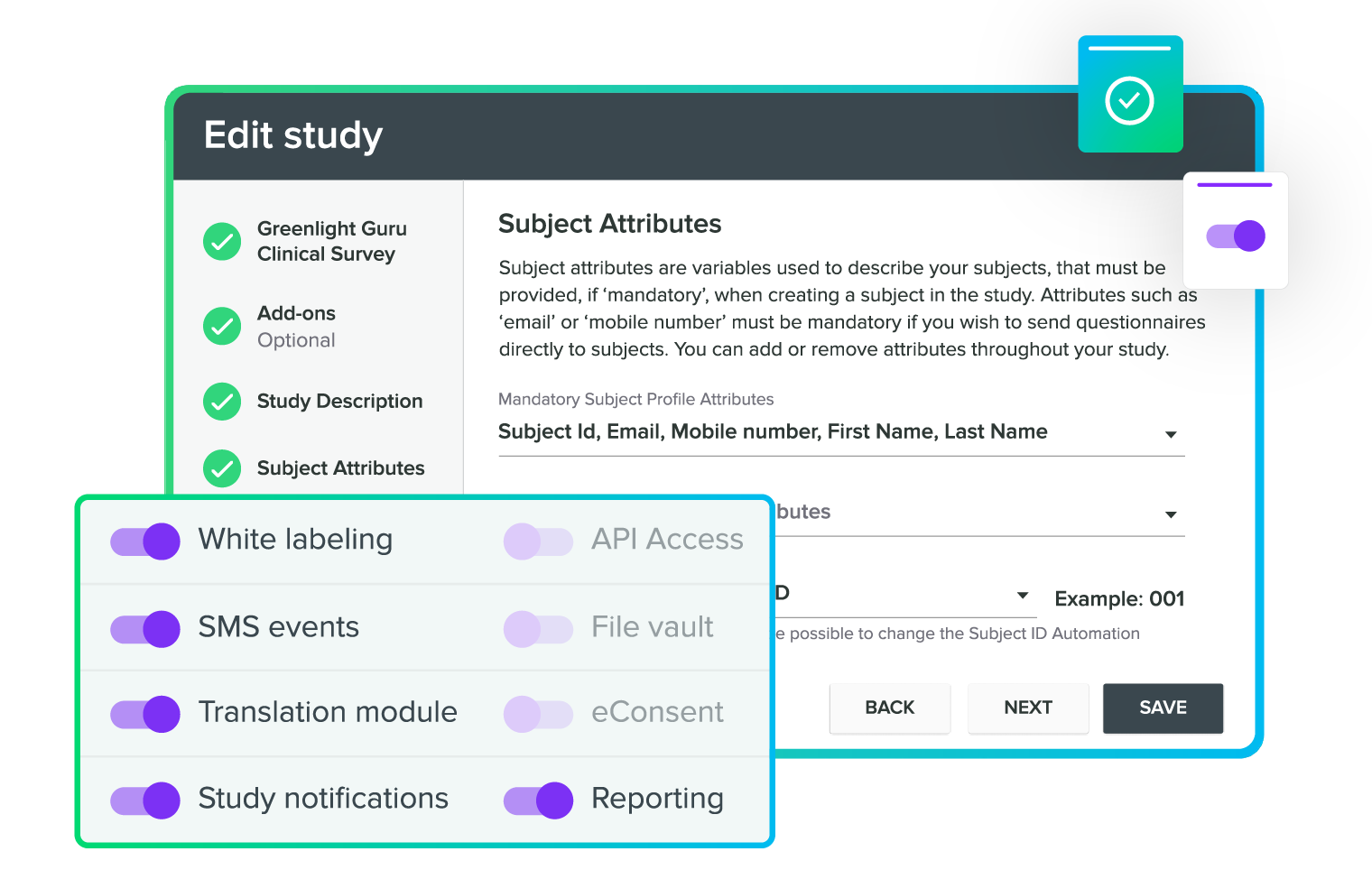
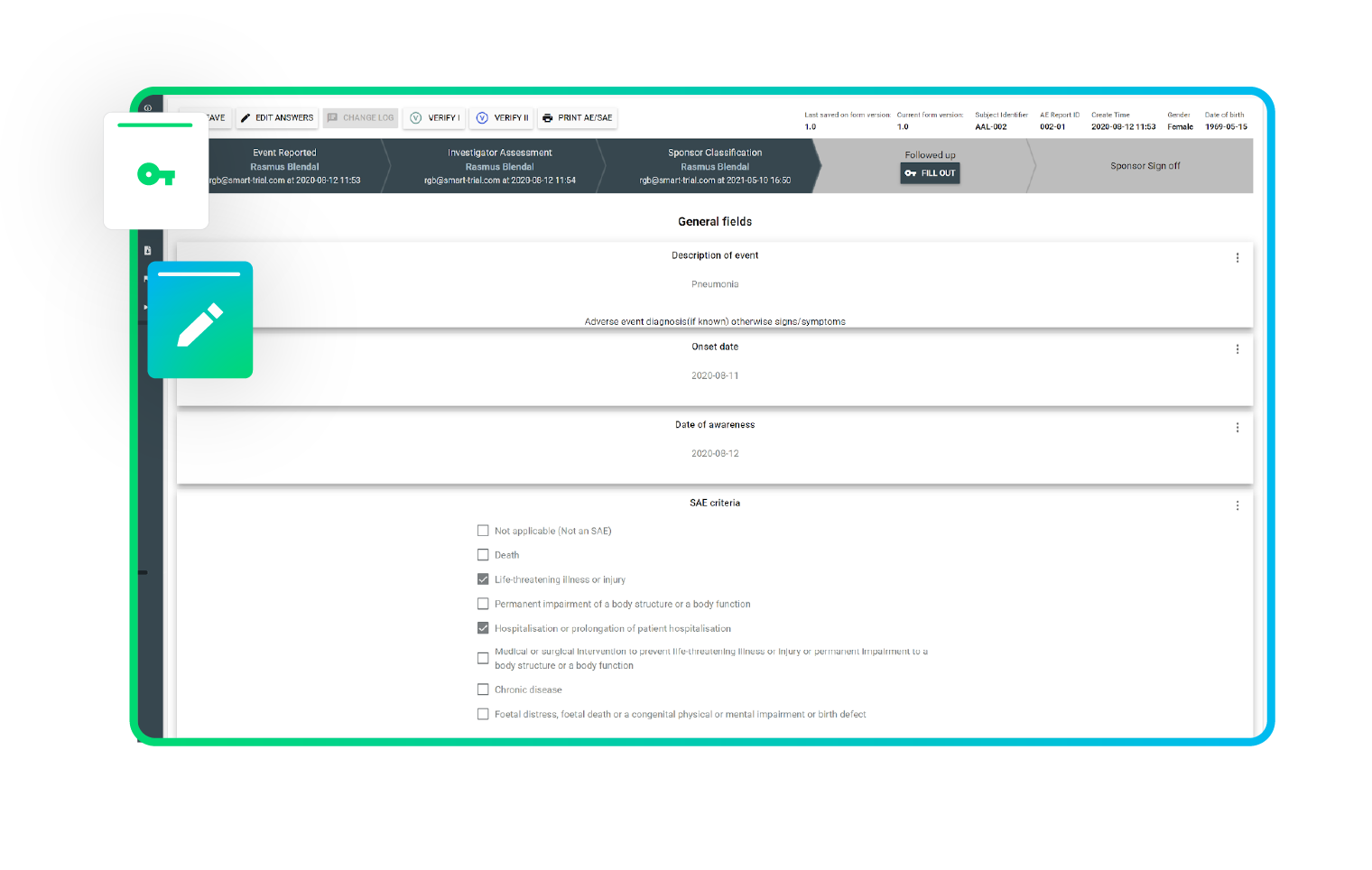
Stay compliant with built-in AE reporting
Easy and secure data transfers
Collect and manage all your clinical data
A single, validated platform with everything you need to run medical device studies
Medical device trials are unique. Greenlight Guru is built to help MedTech companies carry out GCP-compliant studies that generate high-quality clinical data.
Keep your trials on track
 +
+
-1.webp)
— Gheorghe Pop, Medical Monitor & Biostatistician
Study timelines tend to grow. Let’s fix that.
Work in a platform that’s purpose-built to help you start fast, stay on track, and get your data as soon as it’s ready, not weeks later.
Accelerate study
start-up
start-up
Build studies in minutes and reuse existing forms and studies in a validated system that’s optimized for MedTech.
Collect compliant and reliable clinical data
Audit trails, Part 11-compliant e-signatures, and workflows aligned with ISO 14155:2020 ensure your data is submission-ready.
A platform your study sites will love
Build better site relationships with a simple and reliable platform that’s designed for easy onboarding and smooth collaboration.
Fewer errors, cleaner data
Spend less time cleaning data with user-friendly forms that enforce response rules and eliminate potential errors.
Frequently asked questions
What types of studies can I run in Greenlight Guru Clinical?
You can use GG Clinical for early feasibility, pivotal trials, PMA studies, post-market surveillance, registries, and PMCF surveys. The platform supports both pre-market and post-market evidence needs for medical device and diagnostic trials.
How long does it take to set up a study?
Most teams are up and running in weeks, rather than months. Our no-code builder, reusable templates, and dedicated onboarding support help you move fast without needing developers or consultants.
Is your platform compliant with ISO 14155 and 21 CFR Part 11, and GDPR?
Can I use GG Clinical with external CROs or sponsors?
Do you support eConsent, ePRO, and remote data capture?
How do you handle system validation?
What kind of training and support do you offer?
Is GG Clinical easy for sites to use?
Can I reuse forms or studies across multiple trials?
What makes Greenlight Guru Clinical different from other EDC platforms?
Do you have the ability to support tracking of safety events (AE's/SAE's)?







