FDA’s PMA Supplements and Amendments: When and How to Use Each

The regulatory pathway for getting a Class III medical device to market in the US is known as Premarket Approval (PMA). Given that Class III devices pose the highest risk to patient safety, the PMA process is lengthy and requires a large amount of information about the device and the data backing up its safety and effectiveness claims.
FDA must receive and approve a manufacturer’s PMA application before that manufacturer is allowed to market their Class III medical device in the US. And due to the extensive nature of the process, manufacturers often need to change or add information to their PMA. These changes are known as PMA supplements or PMA amendments (depending on the reason for them and the stage at which they are submitted).
Making the decision about whether to submit a PMA amendment or PMA supplement—or which kind of PMA supplement—can be confusing. So, in this article I’ll walk you through when to use each and how to decide which to use.
BONUS RESOURCE: Click here to download a free, customizable PMA Inspection Checklist.
What is a PMA supplement?
A PMA supplement is the submission you’ll use when there is a change affecting the safety or effectiveness of your PMA-approved device. That means PMA supplements are used after your PMA application has been approved. PMA amendments, which we’ll get to later, come before approval of a PMA (or PMA supplement).
It’s important to remember that just like your initial PMA application, FDA must review and approve your PMA supplement before you implement any proposed changes.
What are some changes that require a PMA supplement?
You’ll use a PMA supplement when there is a change to the safety and effectiveness of your Class III device. Some of the changes that require a PMA supplement include:
-
Labeling changes
-
A new indication for use
-
Switching to a different facility for manufacturing, processing, or packaging the device
-
Changes in manufacturing methods or quality control procedures
-
Changes in sterilization procedures
-
Changes in packaging
-
Changes in the performance or design specifications
-
Extension of the expiration date of the device based on testing protocol that FDA has not approved
If the changes you’re proposing do not affect the device’s safety or effectiveness, you may make a change to the device without submitting a PMA supplement. You do still need to report that change in a postapproval periodic report, however. The only exception is for “trivial’’ changes, like the color of your label, which FDA does not require to be reported.
What are the different types of PMA supplements and when do you use them?
Choosing the right PMA supplement for your situation can get confusing, fast. There are many different types of PMA supplements that you could potentially use based on the change you want to make.
I’ll cover them all here, but FDA has also provided a guidance document for choosing the appropriate PMA supplement, which I encourage you to read through on your own. I’ve also added a flowchart based on that guidance at the end of this section, which should help visually clarify your different options. You can also find the regulatory requirements for PMA supplements in 21 CFR 814.39.
Panel-track supplement
The panel-track supplement is meant for changes that require a significant change in the design or performance of your device, or a new indication for use. This is known as the “panel-track” supplement because it requires a full PMA review, which may include a review by an outside panel of experts.
You’ll need significant clinical data to provide reasonable assurance of the safety and effectiveness of your device after the change if you submit this supplement.
180-day supplement
The 180-day supplement is for changes that affect the safety and effectiveness of the device or for significant changes in components, materials, design, specification, software, color additives, or labeling.
This supplement will require in-depth review and approval by FDA, and possibly (but not necessarily) a full PMA review. Generally, the data you submitted in your PMA application will still be applicable for supporting the approval of your requested changes. However, you will likely have to do new preclinical testing on the device for supporting evidence.
Real-time supplement
The real-time supplement is for minor changes to your device, for which you’ve requested a meeting with FDA to review the supplement together. This supplement is appropriate for minor changes to device design, software, sterilization and packaging methods, and labeling that does not affect indications for use or contraindications.
This is one of the more difficult PMA supplements to pin down, so FDA has released guidance on using Real-time PMA supplements that provides more in-depth information on their use.
Special PMA supplement
The special PMA supplement is meant for changes that enhance the safety of the device or the safety in using the device. This is often used for labeling and manufacturing changes that enhance safety, and this is the only case in which the change may be put into place before you get FDA approval of your PMA supplement.
30-day notice and 135-day PMA supplements
A 30-day notice is required for any changes to manufacturing procedures or methods that affect the safety and effectiveness of the device. In this case, you’ll submit a 30-day notice to FDA. After 30 days, you may make the change specified in your notice, unless FDA notifies you that the 30-day notice is not adequate. In that case, the 30-day notice will be converted into a 135-day PMA supplement.
This is another tricky one to wrap your head around, and I would encourage you to check out the FDA’s additional guidance on 30-day notices and 135-day PMA supplements.
Manufacturing site change supplement
You’ll use a manufacturing site change supplement when you want to change the facility or establishment where you’re manufacturing, processing, or packaging the device. You’ll need to submit this supplement if:
-
The change affects the safety and effectiveness of the finished device and
-
The site was not approved as part of the original PMA or a PMA supplement, or
-
The site was approved as part of the original PMA or PMA supplement, but only for the performance of different manufacturing activities.
Note: This is different from the 30-day notice, as the 30-day notice is for changes to manufacturing procedures or methods, not for changes to manufacturing sites.
Finally, if the modifications to your device result in a new device, you will need to submit an entirely new PMA (rather than a supplement) application. FDA considers a change to result in the creation of a new device when the modification results in a design so different from the initial design that it renders pre-clinical and clinical data on the initial device unusable.
All in all, the decision tree for modifying a PMA device looks like this:
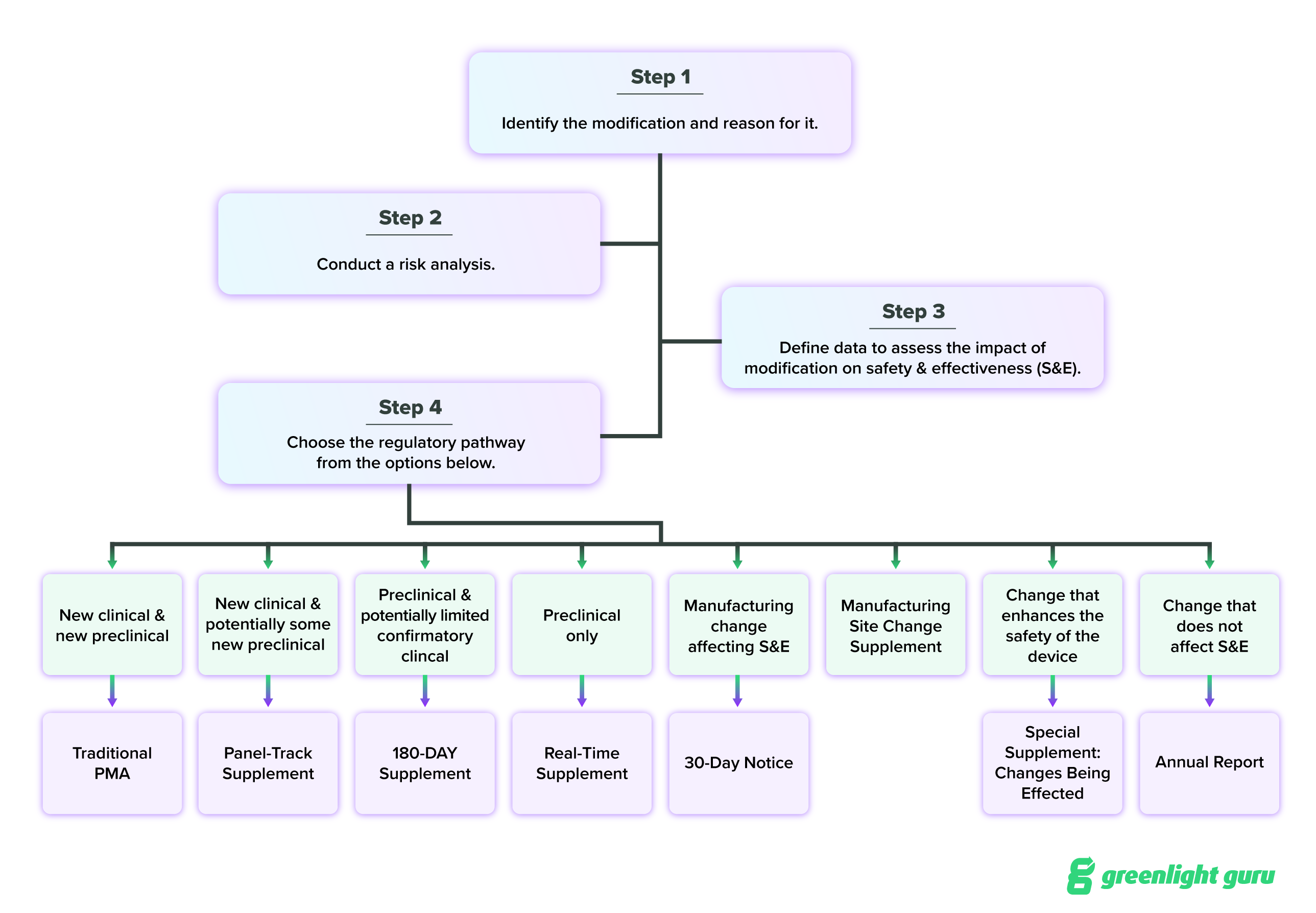
This is quite a bit of information to sift through, and if you’re feeling confused about which PMA supplement to submit, reach out to FDA directly for guidance.
What is a PMA amendment?
A PMA amendment includes all additional submissions to a PMA or PMA supplement before approval of the PMA or PMA supplement. In other words, if you need to revise or add information to your PMA application before it’s approved, FDA wants you to amend the submission.
However, an amendment could also be used on a PMA supplement that has been submitted but not yet approved. For instance, if you submitted a 180-day PMA supplement, and then realized you were missing information in it, you could use a PMA amendment to add that information.
A major PMA amendment can extend the review period up to 180 days. Major amendments include:
-
Significant new data from a previously unreported study
-
Significant updated data from a previously reported study
-
Detailed new analyses of previously submitted data
-
Significant required information that was previously omitted
Your PMA amendment will need to include the reason for its submission and the PMA or PMA supplement number assigned to the original submission.
BONUS RESOURCE: Click here to download a free, customizable PMA Inspection Checklist.
Greenlight Guru keeps all your quality processes & clinical data streamlined, organized & audit-ready
With so much to keep track of, organize, and submit, manufacturers of Class III medical devices cannot afford to be bogged down by out-dated, manual, and often noncompliant tools. Instead, they need software solutions that increase both transparency and speed, ensuring security, traceability, and a single source of truth that’s always audit-ready.
That’s what you’ll get—and much more—with Greenlight Guru’s MedTech Suite. Whether you’re using our purpose-built electronic data capture (EDC) solution to collect the crucial clinical data for your PMA application or managing the entire lifecycle of your device in our QMS software, Greenlight Guru always has you covered.
See how we can help your company get your products to market—and keep them there—with a free demo of our software →
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
QMSR Explained: What FDA QSR & ISO 13485 Harmonization Means for Medical Device Companies
QMSR: The Future of FDA's Quality Management System Regulation for Medical Devices
FDA QSR Transition to ISO 13485: Is Global Regulatory Harmonization on the Horizon?
Get your free download
Premarket Approval Inspection Checklist






