
One of the biggest factors that determines a medical device company’s success is how effectively they manage quality processes.
Still, nearly 50% of MedTech businesses are stuck using a paper-based QMS and disconnected, general-purpose tools.
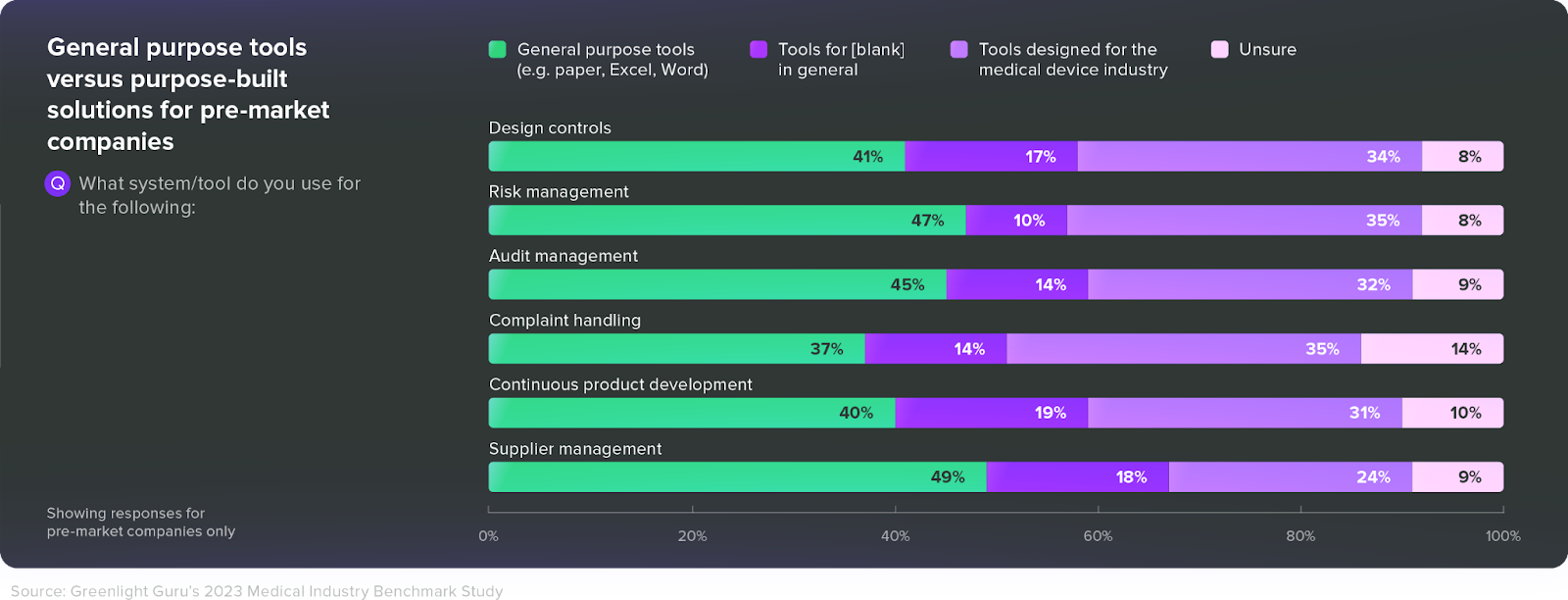
Sometimes, that’s simply because companies feel their paper-based systems are working fine. But in MedTech, “fine” isn’t good enough. If you want to stay audit-ready while producing the safest and most effective device possible, you need to start thinking seriously about how you’ll manage your quality system throughout the lifecycle of your devices.
But don’t just take our word for it. We recently had a panel discussion with several Greenlight Guru customers who spoke directly to the benefits of an eQMS in general and Greenlight Guru in particular.
You can watch the entire webinar for free, but we’ve also compiled four of the key benefits of switching from paper to an eQMS below.
4 benefits of switching to an eQMS
BONUS RESOURCE: Click here to download your free copy of our QMS Software Vendor Checklist.
An eQMS keeps you audit-ready
A paper-based QMS lacks the traceability and tracking capabilities of an eQMS, making it difficult to maintain a complete and accurate audit trail. With a paper-based QMS, you always run the risk of losing or misplacing documents, and tracing a document’s history or validating its accuracy can be downright difficult.
 An eQMS provides a comprehensive and connected source of truth for all quality-related data, including documents, processes, and procedures. Every action taken within the system is recorded and tracked, creating an unbreakable chain of custody that can be audited and traced back to the source.
An eQMS provides a comprehensive and connected source of truth for all quality-related data, including documents, processes, and procedures. Every action taken within the system is recorded and tracked, creating an unbreakable chain of custody that can be audited and traced back to the source.
 And it’s not just MedTech companies that like using an eQMS for audits. Auditors themselves are often impressed by the difference a purpose-built eQMS makes during an audit.
And it’s not just MedTech companies that like using an eQMS for audits. Auditors themselves are often impressed by the difference a purpose-built eQMS makes during an audit.
An eQMS facilitates scaling your business
Whether you’re adding new products, expanding your target market, or moving towards an acquisition, the growth phase for a MedTech company is an exciting time. But if you’re still trying to make due with a paper-based QMS, scaling will cause serious growing pains.
That’s because paper and general purpose tools are rigid and difficult to modify—especially once your operations become more complex. As a result, fewer than 1 in 3 medical device professionals feel very confident that their quality system will hold up while they scale.
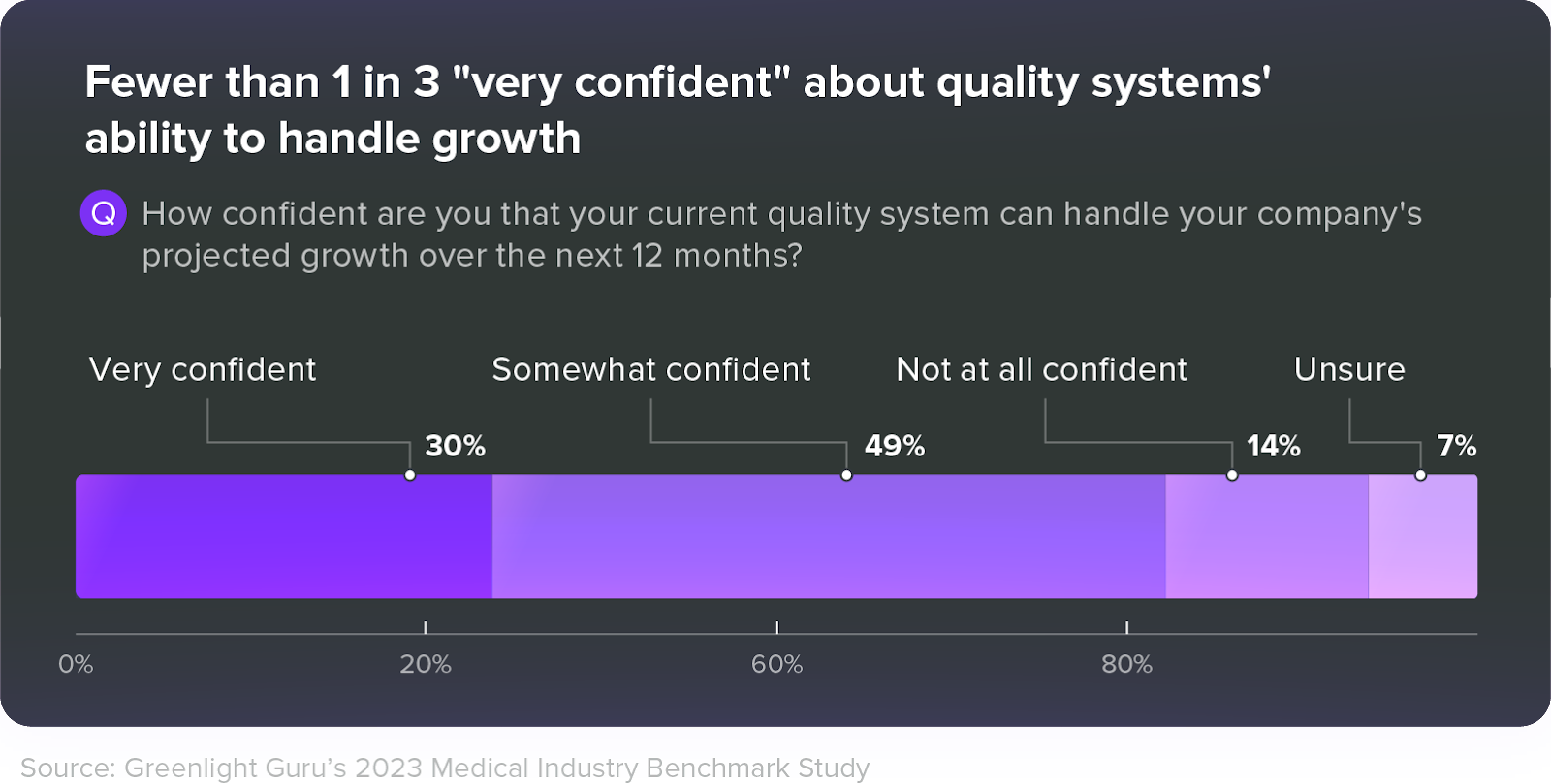
An eQMS, on the other hand, is designed to be highly configurable and customizable, allowing medical device companies to adapt and evolve with ease. Choosing a purpose-built eQMS solution enables growing companies to start automating high-effort tasks and quality processes, such as documentation, internal audits, and maintaining their design history file (DHF).
 An eQMS makes it easy for medical device companies to track when and where signatures and approvals are required, and streamlines the process using automation and electronic signatures. Features like those mean that opening new locations is simply a matter of expanding the number of users, rather than spending endless hours hunting down signatures and trying to standardize processes.
An eQMS makes it easy for medical device companies to track when and where signatures and approvals are required, and streamlines the process using automation and electronic signatures. Features like those mean that opening new locations is simply a matter of expanding the number of users, rather than spending endless hours hunting down signatures and trying to standardize processes.
An eQMS can help you stay compliant with regulations
If you want to find success in the medical device industry, you’ve got to start with the fundamentals—and that means getting your compliance right. Regulatory bodies have strict requirements for how manufacturers are documenting quality processes, and expect you to stay audit-ready to demonstrate compliance at any time.

However, as you switch from paper to an eQMS, you also want to keep in mind requirements that pertain to your new software. Sometimes people are spooked by the idea of switching to an eQMS because they know that both FDA and ISO 13485 require the validation of software (like a QMS) used in the production of medical devices.
It’s true that many eQMS solutions don’t come validated, and the process of validating software in-house requires a large time commitment on your part. Greenlight Guru, on the other hand, comes pre-validated. That means your primary responsibility becomes the installation qualification—a relatively quick process that we provide draft protocols for.
Switching to an eQMS saves you massive amounts of time and money
For some companies, it can be difficult to get everyone on board with moving from an ostensibly cheap system (paper) to one with a significant upfront cost. But it doesn’t take long before an eQMS will start saving you time and money.
 It might be the oldest saying in the book, but time is money. If you want to get your products to market, grow your business, and improve the quality of life for patients around the world, you can be (literally) weighed down by paper. You need an eQMS.
It might be the oldest saying in the book, but time is money. If you want to get your products to market, grow your business, and improve the quality of life for patients around the world, you can be (literally) weighed down by paper. You need an eQMS.
Greenlight Guru’s eQMS is purpose-built for MedTech companies just like yours
A common theme we heard during the webinar discussion was that MedTech companies choose Greenlight Guru for the quality of our eQMS platform, but also because of our focus on their business.
Our team is made up of medical device professionals with decades of combined knowledge in the industry. No matter where you are in your MedTech journey, our team can help guide you on your way.
 So, if you’re ready for more than just another software tool—if you’re ready for the most powerful, purpose-built eQMS for Medical Devices in the world—then get your free demo of Greenlight Guru today!
So, if you’re ready for more than just another software tool—if you’re ready for the most powerful, purpose-built eQMS for Medical Devices in the world—then get your free demo of Greenlight Guru today!
Etienne Nichols is the Head of Industry Insights & Education at Greenlight Guru. As a Mechanical Engineer and Medical Device Guru, he specializes in simplifying complex ideas, teaching system integration, and connecting industry leaders. While hosting the Global Medical Device Podcast, Etienne has led over 200...
Related Posts
Best QMS Software: Ultimate Guide to Comparing Quality Management System Solutions
What is a Modern QMS?
15 Reasons Why Medical Device Companies Choose Greenlight Guru
Get your free resource
QMS Software Vendor Checklist
%20Software%20Vendor%20Checklist%20-%20Slide-in%20Cover.png?width=250&height=321&name=Quality%20Management%20System%20(QMS)%20Software%20Vendor%20Checklist%20-%20Slide-in%20Cover.png)



%20Software%20Vendor%20Checklist%20-%20Slide-in%20Cover.png?width=575&name=Quality%20Management%20System%20(QMS)%20Software%20Vendor%20Checklist%20-%20Slide-in%20Cover.png)



