Improving Study Management in MedTech with Greenlight Guru Clinical’s Custom Collaborator Roles

Greenlight Guru Clinical's recent introduction of Custom Collaborator Roles provides vital support to the MedTech clinical study arena. This feature brings a targeted solution to the challenges of managing large, multi-site clinical trials and surveys, focusing on the intricate needs of user roles and permissions management across many users.
We have worked closely with customers and key stakeholders to rethink our approach to user roles and permissions. This ensures that larger, more complex studies are executed with efficiency and precision. Scalability is crucial for MedTech companies aiming to undertake extensive pre and post-market studies across various geographical locations.
The introduction of Custom Collaborator Roles significantly reduces the time related to initiating and managing studies while simultaneously enhancing safety by minimizing the risk of human error in access control and role management.
A feature by the industry, for the industry
Central to the development of this feature was an extensive collaboration with many of our customers. This collaboration ensured that the final product is an exact fit for the industry's unique demands, offering a high level of usability and flexibility for both small and large studies.
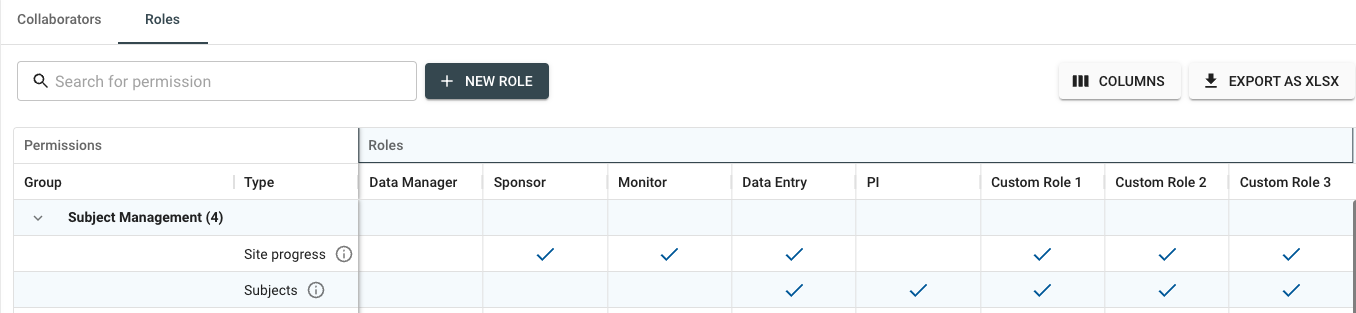
The Custom Collaborator Roles feature provides a more user-friendly interface that allows for quick, multi-site setup and offers an easier overview of user permissions through named roles.
This also facilitates better management of studies and streamlines the process of demonstrating compliance to auditors, with the ability to export permissions and roles directly to Excel.
Optimize your study management workflow with Greenlight Guru Clinical
The release of Custom Collaborator Roles is a testament to our commitment to innovation and dedication to supporting the MedTech community. We continue our extensive collaboration with the industry to lead the way in clinical data management solutions, ensuring that Greenlight Guru customers are one step ahead.
Interested in learning more about Greenlight Guru Clinical, the leading EDC system made specifically for Medtech? Book a customized demo with our team and we’ll show you how to efficiently manage user permissions to streamline your clinical study management workflow and reduce setup times in your specific study.
Páll Jóhannesson, M.Sc. in Medical Market Access, was the founder and former CEO of Greenlight Guru Clinical (formerly SMART-TRIAL) and is currently the EVP of Europe at Greenlight Guru.
Related Posts
Ultimate Guide to ISO 14155:2020 for Medical Devices
10 Best Practices for eCRF in Medical Device Trials
Everything You Need to Know About Electronic Data Capture (EDC) for Clinical Trials
Get your free DEMO
Clinical Electronic Data Capture (EDC) Software




