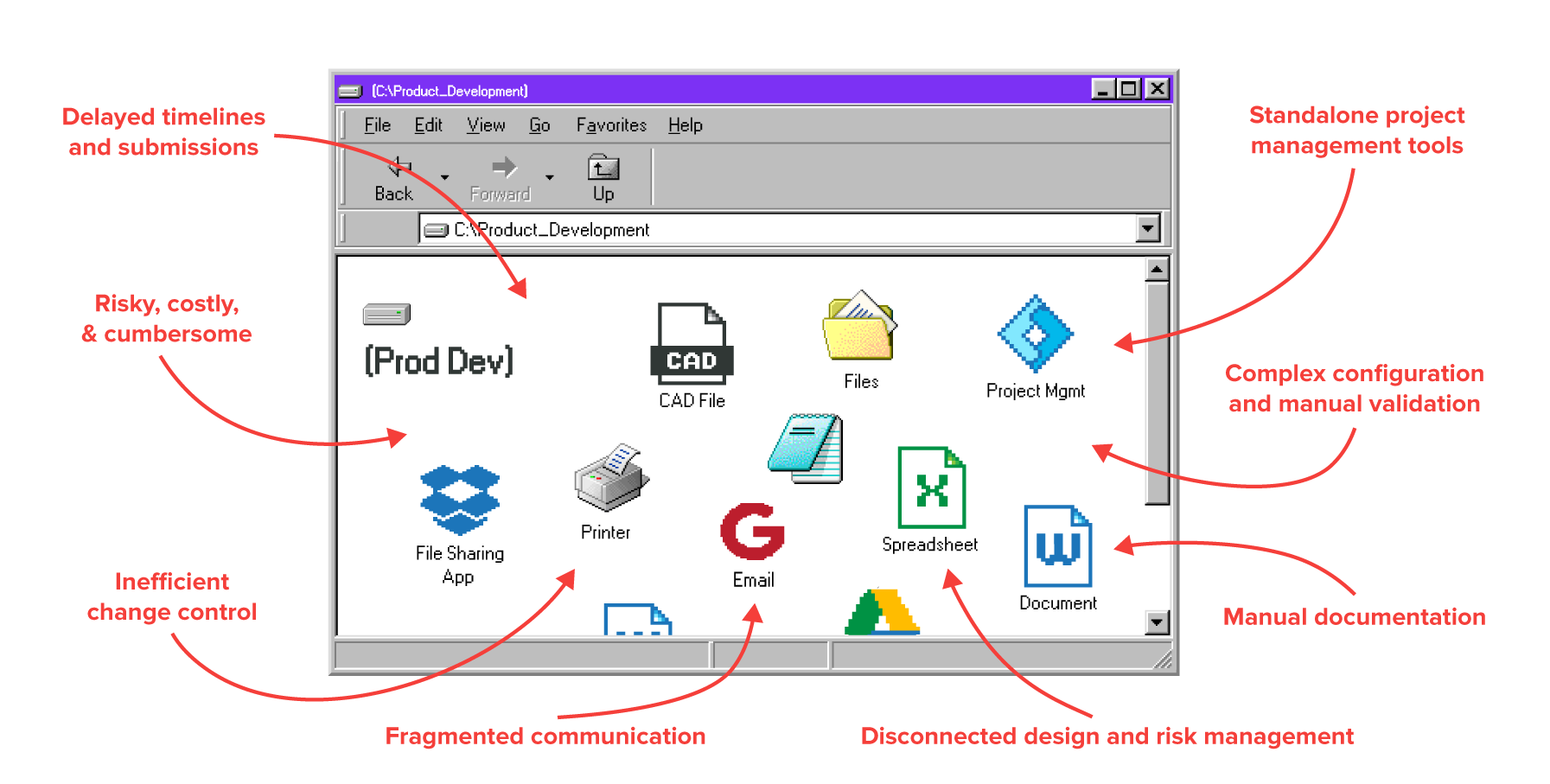
Delay Development.
creating life-changing innovations.
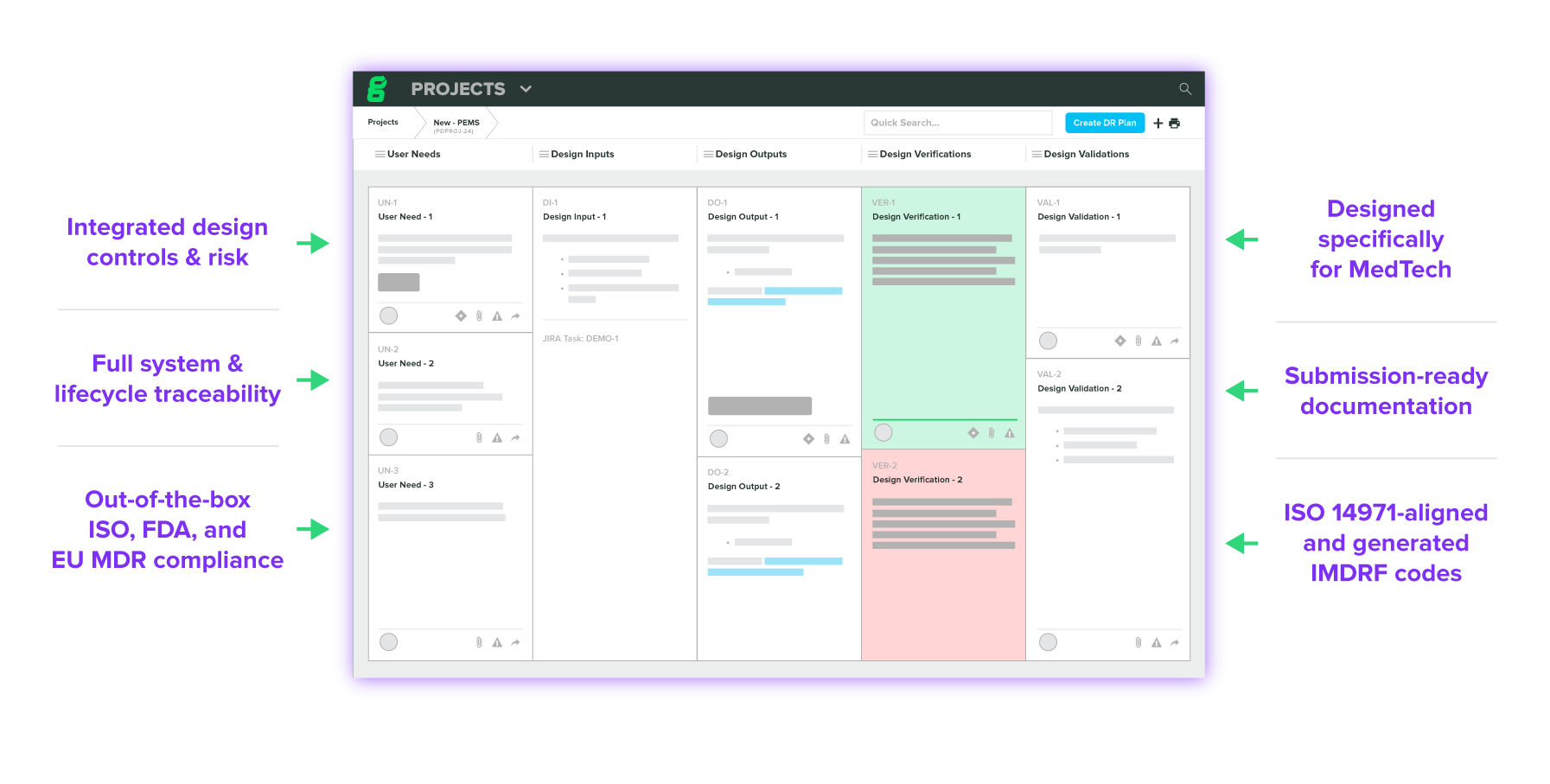
Engineered Specifically for MedTech
Your tools should be optimized for MedTech's intricate compliance requirements. Streamline your development process and automate compliance to accelerate your timelines.
Advanced design control and risk management with built-in compliance. Intuitively document device requirements and risks while you design, build traceability, and maintain living device records.
Reduce time spent in spreadsheets and binders. Our solution enhances traceability, teamwork, and accountability, ensuring up-to-date, accessible information.
Discover, mitigate, and prevent potential risks as early as possible while ensuring that risk management is a living process throughout the entire product lifecycle.
Features that Make Innovation Efficient
Document, track, and trace all aspects of your design and development to bring high-quality devices to market faster.
Easily manage multi-level device requirements in your traceability matrix and generate a completed Design History File on demand.
Build your risk acceptability matrix with the only cloud-based risk management solution that aligns with ISO 14971:2019. Demonstrate a risk-based approach to design with full traceability to related Design Controls.
Learn MoreCreate and update your traceability matrices and schedule design and risk review with Part 11 compliant workflows in mere minutes — not hours or days.
Eliminate the guesswork with personalized insights to predict risk identify and predict the most relevant hazards and harms generated from real-world adverse event data.
Instantly trace and retrieve design records linked to your activities enhancing visibility and access to key design and risk documentation.



















