Products


Featured Capabilities:

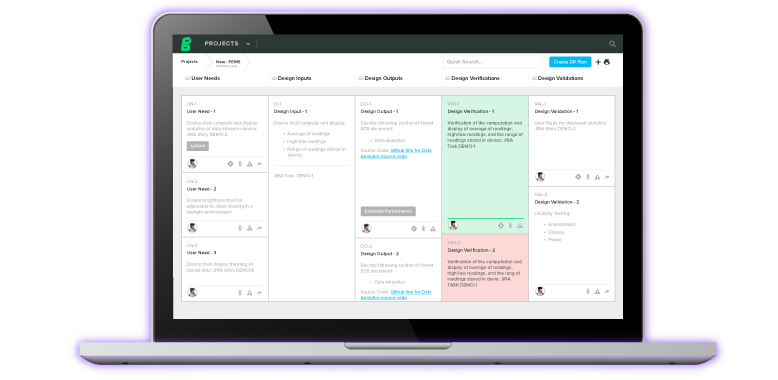
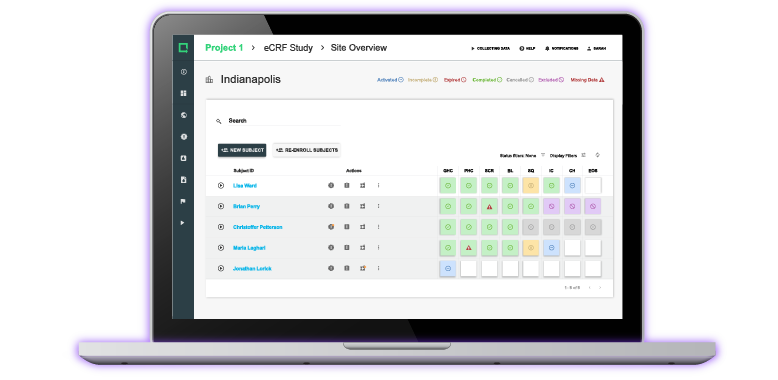
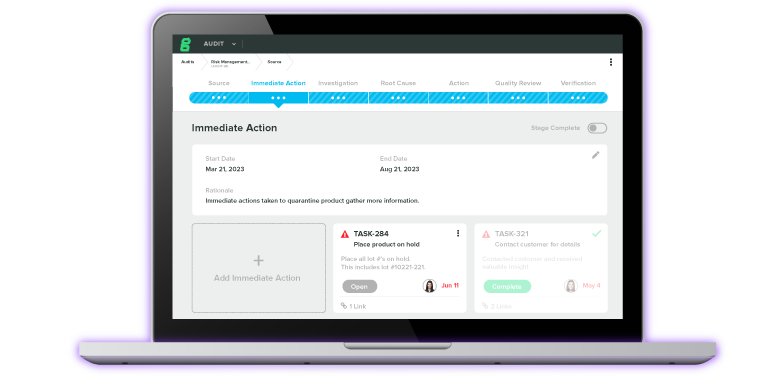
Experience the #1 QMS software for medical device companies first-hand. Click through an interactive demo.
View Product Tour