How To Navigate the Difficult Road of Medical Device Product Development While Avoiding the Common Pitfalls
The story I’m about to tell you is about Eddy Engineer and his trials and tribulations of bringing a new medical device to market.
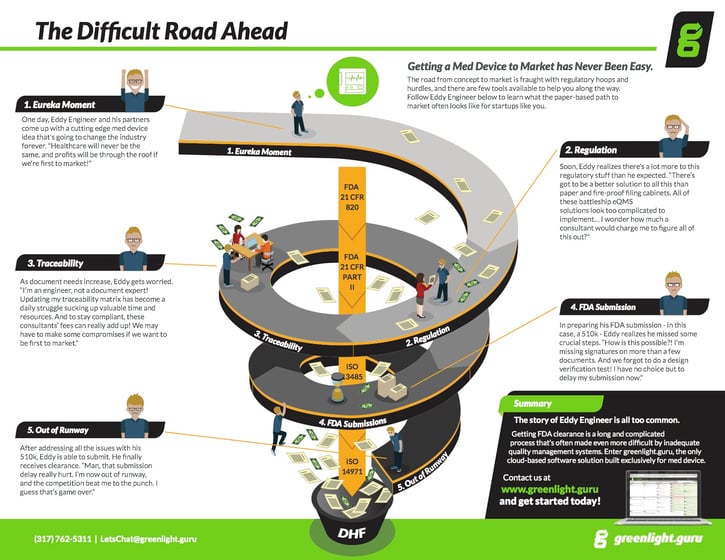
1. The Eureka Moment
Have you ever had or been part of a new and exciting idea? An idea for a medical device product that can improve and save lives?
Meet Eddy Engineer. Eddy recently came up with a brand new idea for a medical device.
This “eureka moment” is definitely one of the most exciting feelings in the world. To be a part of developing a new medical device that will help people is one of the most thrilling moments Eddy has ever had.
Eddy gets to work, building prototypes to demonstrate his proof of concept. He gets feedback on these prototypes from the doctors and nurses who will eventually use his medical device.
Eddy and his team conduct market research to determine the overall need and market opportunity. The team also does some competitive analysis to evaluate who else is trying to solve this issue.
After all that, Eddy knows he is really onto something.
2. Medical Device Regulations
Eddy knows his idea is a medical device and that regulations will have an impact on your ability to go to market. But Eddy has not done much research yet on the impact of all these regulations.
Eddy is not discouraged.
He discovers information about FDA product classification, as well as how to determine product classification in EU.
Eddy decides to focus on the U.S. market initially and starts to understand what a 510(k) submission is—since this will be required to get FDA market clearance.
With the compelling market need, proof of concept supporting evidence, and regulatory pathway identified, Eddy is successful in raising some funds to turn his medical device idea into reality.
Eddy eventually discovers FDA quality system regulations and this thing called ISO 13485. He realizes that he will need to establish a quality system to support his medical device.
Eddy starts to realize there is a lot more to the regulations than he thought.
He does some research on eQMS software solutions and finds a few options to consider. Eddy schedules a few demos of the well-known eQMS options.
Unfortunately for Eddy, he quickly discovers that these tools are way over built for young medical device companies like his. He realizes by the time he’d implement one of these legacy eQMS solutions, he very well could be out of runway.
Plus, most of the eQMS options available are too expensive and not designed for the medical device industry.
Eddy also considers bringing in a consultant or two to help. Leveraging experienced resources should help, however, Eddy didn’t budget for this.
Oh well, he knows he has to take this step. Quality system regulations are the absolutely a must for a medical device company.
3. Traceability – Making sure it all gets done
As product development progresses, Eddy is excited to be a hands-on engineer. Designing his product is exciting. Building and testing prototypes is really fun!
And then Eddy’s consultants remind him of the need to document and address Design Controls.
Fortunately, Eddy discovers “The Ultimate Guide to Design Controls”, which helps him better understand all these things like User Needs, Design Inputs, and Design Verification.
But the Design Control and quality system implemented by his consultants is paper-based and painful to use.
Eddy gets the need to document traceability of all his Design Controls; he just wishes he didn’t have to use spreadsheets and paper to do so.
4. Time for an FDA Submission
Thankfully, the consultants took on most of the traceability and documentation needs for Eddy’s project. This freed him up to work on the fun stuff—prototyping, designing, and testing.
Eddy realizes his monthly expenses have increased since he asked the consultants to manage much of the documentation. He still needs their help but decides to take the lead on putting the 510(k) submission together.
This 510(k) submission is critical. Eddy knows it has to be right in order to get clearance from FDA. Eddy dives into the piles of documents and takes ownership of the traceability spreadsheet.
He goes through every single User Need, Design Input, Design Output, and Design Verification activity to ensure that every single item has been addressed and is supported with objective evidence.
Except he discovers a few gaps—things that have not been done. Things that absolutely must be addressed before sending to FDA for review.
Eddy is torn. He does not want to delay the 510(k) submission and he knows he cannot send this to FDA without it.
So, the 510(k) is delayed and the gaps are addressed.
More time. More expenses.
Eventually, Eddy addresses the gap in his Design Controls, finishes preparing the 510(k), and submits to FDA for review.
5. Out of Runway
The FDA 510(k) review process is a bit painful. Eddy’s consultants did warn him that FDA almost always has questions.
And they did. FDA also asked Eddy to provide some additional documentation and testing in order to support some of his product claims.
Eventually, Eddy receives a coveted market clearance letter from FDA.
He wants to be excited about this. However, two larger, well-funded competitors launched new products similar to Eddy’s a couple weeks ago.
And Eddy is out of funding to scale up manufacturing and product launch activities.
Avoid the Common Pitfalls
As you look back over Eddy’s story, I suspect you can relate to a few of the pitfalls he went through taking a product idea through the medical device product development process.
Perhaps you are dealing with similar issues.
Let me provide you with three points that will improve your medical device product development efforts.
#1: Traceability of Design Controls Should Be Easy to Manage
Without a doubt, one of the single biggest challenges you are faced with during medical device product development is Design Controls.
And more specifically, you absolutely have to keep traceability of these Design Controls in mind from day 1!
In Eddy’s case, he had a spreadsheet to keep track of traceability.
Traceability is so vital because it provides the proof and evidence that you have addressed all Design Controls. Traceability provides the linkage to show how User Needs are validated and Design Inputs are verified (and everything in between).
Using a spreadsheet to keep track of all the User Needs, Design Inputs, Design Outputs, Design Verifications, and Design Validations is possible. Possible you are detailed oriented and diligent about keeping traceability up to date and current every single day of the project.
Or you can use a better solution for ensuring traceability—a solution designed specifically for the medical device industry. A solution designed with helping you ensure traceability from day 1 of your medical device product development efforts.
#2: Quality System Should Be Designed to Scale
When Eddy realized the need to establish a quality system, he tried to find a tool to help. Many of the options he found are clunky and difficult to use, which are not designed for the medical device industry and require months to implement.
Legacy eQMS tools are not a viable solution for small to mid-sized medical device companies.
Eddy decided to leverage the advice of his consulting team and implemented a paper-based quality system. A paper-based QMS option is difficult to keep current and up to date.
Neither is really a good option for a medical device company. Neither will free you from a quality system nightmare.
Your quality management system should be designed in a way that can easily scale. A QMS should always evolve to meet the needs of your medical device company. FDA 21 CFR 820 and ISO 13485 should be the foundation of the QMS solution you use.
#3: Own the Responsibility
Consultants can be great resources to help your medical device company with everything from Design Controls, to regulatory submissions, to quality systems (I should know, I’ve been consulting since 2007).
Eddy ultimately decided to leverage the expertise consultants can bring to the table.
Except Eddy did not ultimately own the responsibility for Design Controls and the quality system. He let the consultants drive and make the decisions.
At the end of the day, this is your medical device company. You need to take control and make the decisions that are necessary to bring new products to the market.
This article originally appeared as a guest post on Qmed and is being republished here with permission.
Jon Speer is a medical device expert with over 20 years of industry experience. Jon knows the best medical device companies in the world use quality as an accelerator. That's why he created Greenlight Guru to help companies move beyond compliance to True Quality.