How to Translate Voice of Customer into User Needs

How well do you do at capturing “voice of customer” and translating into user needs?
Whenever you’re embarking on the journey to creating a new medical device, one of the key things to establish from the outset is your user needs. These are important for two key reasons:
- Once you have user needs defined, then you can establish clear, objective, measurable design inputs.
- Once you've developed your medical device, then you need to prove it meets user needs via design validation.
The challenge at the beginning is clearly establishing those user needs in the first place. Talk to 30 people about a perceived problem or need and you will probably get 30 different answers or customer “voices.”
How can you get clear user needs through all of this? Let’s look at a few tips:
Free Bonus Giveaway: Need to survey your users? Here are 6 tools that you can use.
What is “Voice of Customer”
What the customer believes that they need and/or want. Your device is created to meet a need, serve a purpose, and solve a problem.
Sometimes a customer isn’t really able to articulate the problem statement, they often know what it is that they feel they need though, so part of our job as medical device developers is to talk them through and identify that core problem statement. We translate their thoughts to user needs to ensure we’re making the right device.
What are user needs?
These are the articulated problems that you need the device to solve. They’re usually generic; for example, a wearable device might need to be “lightweight” without any specifics around weight.
Capturing user needs
It can be challenging to translate that “voice of customer” into user needs, so where do you begin? It often starts with a simple interview with someone you know, perhaps a physician who is close to the problem.
You might do this informally as a candid conversation, just to get some initial thoughts and ideas about what those needs might be.
It’s always a good idea to seek more than one opinion - you should never be building a medical device with one person in mind. Other strategies to gather data might include more formal techniques, such as surveying or focus groups.
You might also decide to shadow someone in the field, where appropriate. You will often get a much better idea of the mechanics of processes and user needs if you can actually observe them at work. Even if you don’t yet have a solution to their problem, observing them is often enough to trigger ideas.
For products on the market
It’s always a good idea to be gather feedback, even for products that you already have on the market. Sometimes, your first version isn’t necessarily the best it could be, so capturing user needs through your feedback or complaints processes is a good way to stay on top of possible improvements.
For products already on the market or those in development, another option is to create a prototype, give this to the customer and gather their feedback. Overall, you might get feedback throughout different iterations of your product.
Problems with translating user needs
One of the chief problems is that people often think you can just go out and start talking to customers adlib. It is always better to create a process for doing so first, otherwise you can run into any of a few challenges, such as:
- Failing to identify all customer groups. Think about end-users, patients, staff, hospital admin, and any others who have a touchpoint with the device. You want feedback from them all because you need to understand how they are impacted.
- Sometimes the customer has already worked through a design solution in their mind. They’ve jumped ahead and they think they know what needs to be specified for the design of the device. For example, they might think that a “lightweight” wearable should be one pound, even though they’ve never worn a one pound device themselves! Any details like this need to be left up to your product engineers.
- The customer and the engineer don’t work together to tease out needs vs. wants. What is really required and what is simply a “nice to have?” There will be a tendency of customers to throw in all bells and whistles, but have uncertainty about the differences between the two. Be clear about your process to that it is productive for both the customer and your engineer.
- You might use an inappropriate method to gather information. Your methodology needs to be suited to gathering the type of information you need, so having a defined process is always helpful for identifying this.
- The customer might give a very broad user need which you need to whittle down to specifics. You will need to have a process to understand this and to make sure you really have deciphered some clear needs.
A best practice process
There’s a big difference between the broad category of user needs to the specifics of design inputs, so you want to be careful with this. At this stage, you are not looking for design inputs as those are to be left to the engineers once needs have been identified.
For example, if the customer says they’re looking for good customer service, you need to break that down to specifics of what that means without design specifications. This particular example isn’t necessarily about the design of your device, but broken down further might include indicators such as “representatives are knowledgeable.”
The diagram below gives an example of what “comfortable” might mean when broken down into more specific user needs:
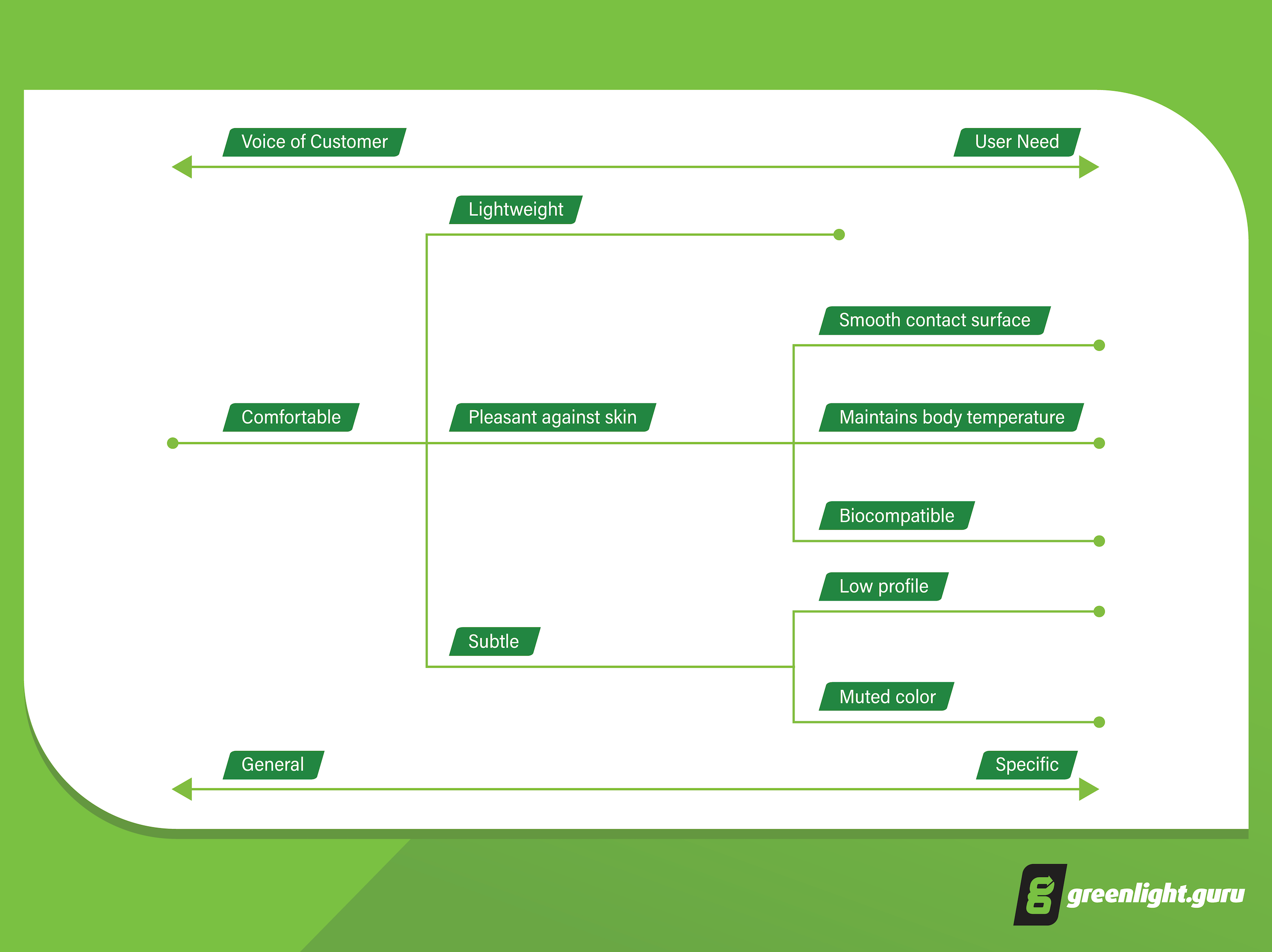
Notice how you can break down needs into further specifics, while stopping short at an actual design specification? Subtle might mean “low profile” and “muted color”, but we haven’t stated how high the profile should be or what colors.
To help you get clarity on the information you might want to extract from your users, we put together 8 questions that define medical device user needs.
User needs vs. design inputs
A user need is a generic requirement, such as lightweight, whereas the design input is a specification you can measure (e.g. “the device weighs 0.5 pounds.”). This gets verified in your design validation stage, which is designed to answer the question, have we made the right device?
You would go back to your customer and say, “does this feel lightweight to you?” So basically, you’re using the customer as part of your validation, too.
FDA backs the idea that all design controls should begin with your user needs and cascade down into other stages of the process. It’s therefore important that you document everything you’ve observed as a need, even if it seems obvious to you. This helps to ensure that everyone involved in the process has the same overall vision and you’re all working toward the same goals.
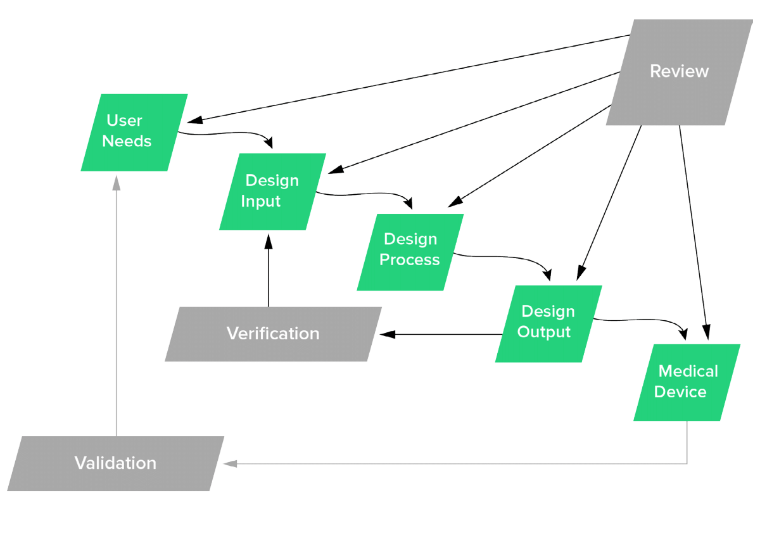
Benefit of getting voice of customer right
You’ve just gone through several months, maybe years of development prior to hitting the market, so it’s important to go back and validate your design with them. Did you capture customer voice well? If you haven’t got it right on the front end, you will have potentially wasted a lot of time.
Free Bonus Giveaway: Need to survey your users? Here are 6 tools that you can use.
Final Thoughts
Translating that “voice of customer” into user needs is one of the most important tasks you can undertake early in your design process.
Key pointers are:
- Have a clear process for gathering user needs.
- Be clear about the specifics of user needs without straying into design inputs.
- Identify all key customer groups.
- Keep gathering feedback throughout the process and revisit the user once you have a working model.
Get clear on your user needs in the first place and you can save a lot of wasted time.
Still using a manual or paper-based approach to manage your design controls or quality processes? Click here to learn more about how Greenlight Guru's modern eQMS software platform exclusively for medical device companies is helping device makers all over the globe in more than 50 countries get safer products to market faster with less risk while ensuring regulatory compliance.
Nichole is a medical device quality and regulatory professional and a Solutions Consultant at greenlight.guru.
Related Posts
A Guide to Bridging User Needs Into Design Requirements
How to Define User Needs During Design Controls
5 Tips for Medical Device Engineers on FDA Design Controls
Get your free resource
6 Tools to Help You Survey User Needs





