Before you start, you should clarify the following with your Notified Body:
Will your Notified Body be certified by MDR?
Yes: Save your MDR audit date!
No: Change your Notified Body as soon as possible!
The European medical device industry will undergo significant changes as a result of the new medical device regulation which has signed on May 26th, 2017. As the name suggests, is it a regulation and no longer a directive and all medical device companies that sell medical products in Europe have to adhere to this new regulation.
Companies that do not follow this regulation will no longer be allowed to sell their medical products in the European Union after May 26th, 2020. Depending on your role as economic operator (manufacturer, importer, authorized representative, distributor) the impact can be meaningful.
Below you will find a step-by-step implementation guide with regards to the new medical device regulation (MDR EU2017/745). Our guide is simple to understand and will allow you to save time and money when implementing the new European MDR regulation.
Note: This guide should not be considered as a recommendation, it’s just based on our own experiences.
Before you start, you should clarify the following with your Notified Body:
Will your Notified Body be certified by MDR?
Yes: Save your MDR audit date!
No: Change your Notified Body as soon as possible!
In a first step you should check if the new MDR rules have any impact on your existing or future products classifications.
We recommend using our Classification Form.
Depending on your business you may have one or multiple economic operator responsibilities, which is imperative to know before you start with the gap assessment. Economic operators are Manufacturers, Importers, Distributors or Authorized Representative. Each of them has different requirements to fulfill per the MDR.
Our Economic Operators Tool will help you to get a better understand of each operator and what their responsibilities are.
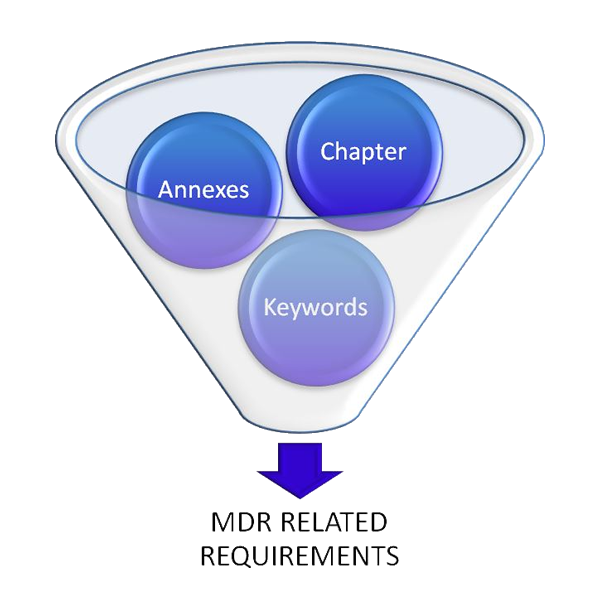
Reduce not required information by going through the chapters and annexes and eliminate all not required information.
Define keywords which are not applicable to you. In our MDR tool you have the opportunity to search for these keywords.
Go through all open requirements step by step and define if requirements are relevant for your business or not.
With our MDR Gap Analysis Tool you are able to do all these steps above and you can reduce the information immediately.
Prior to starting the implementation phase, you should put a plan in place. The steps below will guide you through the main topics.
Our General Safety and Performance Checklist stores the complete requirements of MDR annex 1.
Technical file update according to the MDR requirements.
Your risk management activities (plan, analysis, and report) should be aligned with your PMS and PMCF activities.
Each medical device needs a UDI-DI (Unique Device Identification – Device Identifier) and UDI-PI (Unique Device Identification – Production Identifier) and must be submitted and transferred to the UDI database. (See document from the EU Commission)
Post market surveillance is defined in chapter VII of the MDR.
Manufacturers shall conduct a clinical evaluation in accordance with the requirements set out in Article 61 and Annex XIV, including a PMCF.
Manufacturers shall ensure that the device is accompanied by the information set out in Section 23 of Annex I in an official Union language(s) determined by the Member State in which the device is made available to the user or patient. The particulars on the label shall be indelible, easily legible, clearly comprehensible to the intended user or patient.
The Commission concluded that it will only be possible to make EUDAMED operational once the entire system and its different modules have achieved full functionality and have been subject to an independent audit. Therefore EUDAMED’s launch will be done together for medical and in-vitro medical devices, at the original date foreseen for in-vitro medical devices i.e. May 2022.
The date of application of the MDR remains May 2020.
Take care about upcoming specification updates. We keep you informed with our Regulatory Intelligence Paper. Furthermore, this paper helps to address regulatory activities according to ISO 13485:2016, Chapter 5.6 Management Review.
Performing internal audits and a final mock audit to ensure the key requirements have been implemented.
Make a final "written" checklist, where you can show the evidence for each requirement. It helps you during your next audit. We recommend to do it directly in your MDD vs. MDR Gap Assessment Tool.
The final checklist will ensure the completeness of your implementation process.
Looking for a design control solution to help you bring safer medical devices to market faster with less risk? Click here to take a quick tour of Greenlight Guru's Medical Device QMS software →
Regulatory Affairs Expert in the medical device field. Founder and CEO of Regulatory Globe, a consulting company that offers regulatory tools and resources to medical device companies, specifically in the areas of Medical Device Regulation / EU MDR, In Vitro Diagnostic / IVDR, ISO 13485: 2016 and MDSAP.

Solutions built for medical device teams to move faster, stay compliant, and grow with confidence.
Manage quality events, training, and documentation in one place.
Control documents, training, and change with traceability.
Track CAPAs, audits, and NCs with audit-ready records.
Qualify suppliers and manage parts with visibility.
Connect design, risk, and AI-powered traceability as you build.
Link needs, requirements, and verification to design controls.
Automate traceability from dev tools for faster releases.
Assess ISO 14971 risk as requirements and tests evolve.
Oversee studies with connected data, documents, and workflows.
Capture compliant clinical data with a validated medical EDC.
Plan and manage PMCF aligned to EU MDR expectations.
Collect clinical outcome data in-person or remotely on any device.
Built with regulatory insight to help medtech teams move faster with confidence.
Find issues faster and make smarter decisions with less manual work.
Tools and resources that help teams implement quickly and meet requirements.