Electronic Data Capture (EDC) Buyer’s Guide: Making the Business Case for a New EDC System
Whether you’ve got a new clinical trial on the horizon, or you’re just fed up with the limitations of the way you’re currently capturing clinical data, you’ve probably got a new electronic data capture (EDC) system on your mind.
But there’s a catch: you can’t just swipe the company card and add a new EDC system to your tech stack. This is a significant decision with associated risks and financial implications for the business.
Instead, you’ve got to convince the executives who hold the power of the purse strings to authorize the purchase of a new EDC system. And to do that, you’ll need to build a strong business case—something you can present to show you’ve done your due diligence, have a plan for making the transition, and can clearly speak to the benefits of this investment.
This process can feel daunting if you’ve never done it before—and even if you have—which is why we’ve created this guide. We’ll walk you through the process of building the business case for your new EDC solution, pitching it to management, and making the transition once you have approval.
Like any big project, you just have to take it step by step.
Table of Contents
 1. Find out your organization’s internal purchasing process
1. Find out your organization’s internal purchasing process
Your company probably has an internal process for a large purchase like an EDC system. In midsize-to-large MedTech companies, you’ll likely be able to find this process in the finance department, or perhaps in a dedicated purchasing department that operates under finance’s umbrella.
However, if your company is smaller, the “process” may consist of convincing one or two executives that this purchase is necessary and will end up benefiting the business.
Whether you’re following a defined purchasing process or not, the first thing you need to do is lay the groundwork for the business case you’ll be making. That means answering questions like:
-
Is there a policy on vendor evaluation? How many vendors are you required to evaluate?
-
Do you know the current budget for an EDC system? If not, can you find out how much can be allocated? Will that need to increase for this purchase?
-
Where in the budget cycle are you? Has a budget already been approved for the coming year? Timing can play a large role in getting approval for a new EDC system.
-
Do you have upcoming time-sensitive milestones like a clinical study on the horizon that you’d like to use this system for?
-
What documents will you need to create and what other information will you need to gather to build your business case?
-
Whose final approval will you need for this purchase? Is it a department head? Finance? The CEO? Will there be several people who need to sign off on this?
Write out all the answers to these questions and anything else you learn about your company’s purchasing process. Keep that document handy as you work through the process.
In a smaller organization, there may be no established purchasing process, but you’ll still benefit from asking these questions as you build your business case for this purchase.
![document-organization-needs-eqms-guide]() 2. Document your organization’s needs
2. Document your organization’s needs
You clearly know why you need a (new) EDC solution, but that doesn’t mean your department leader, CFO, or CEO does.
One of the essential parts of any business case is defining your organization’s needs and clearly articulating the goals and objectives of your proposed solution.
This will be at the heart of your business case, and it should answer questions like:
-
What are the challenges you face with the way you’re currently collecting and managing clinical data? How are these challenges affecting the business?
- For instance, is your current clinical data solution contributing to data quality issues or problems with the staff at study sites? Is it burdensome to set up studies with your current EDC system? Are you compliant with regulations on data collection and management or clinical standards like ISO 14155:2020?
- If you don't currently use an EDC system, what challenges do you face with paper, Excel or other data collection tools? Do you struggle with data security and compliance risks? Is the lack of real-time insight into your data causing delays or hurting the quality of your data? Do you spend longer than you’d like cleaning data and preparing for analysis?
- How is your current system helping you adhere to the EU MDR, GDPR or HIPAA, FDA’s 21 CFR part 11, and ISO 14155:2020? Ask yourself the same question if you were to use paper or Excel to collect clinical data
-
How is the organization operating with the current solution, and how would a new EDC solution change operations for the better? What are the benefits of switching?
-
How are you going to measure success? What metrics will you use? What OKRs (objectives and key results) will you be tracking? E.g., time spent on entering data or preparing it for analysis, or survey fill-out rates.
If you have supporting data related to costs, document that here. Your main goal here should be to clearly and concisely prove why your organization needs a new EDC system.
You’ll also want to show your department leadership or executive team that a new EDC system will provide measurable value to the company and there will be a clear return on the investment in the software. You can frame this in a number of ways.
For example:
-
If you choose an EDC system made specifically for medical devices and diagnostics, you’ll save a large amount of time setting up studies as all features are optimized for MedTech studies out of the box. Additionally, MedTech-specific systems typically cost far less than a pharma-specific EDC.
-
The new EDC system will enable you to collect high-quality data thus ensuring you a more streamlined pathway to regulatory submissions and go-to-market which in turn will generate more revenue for the company. This is especially important to point out if you’re currently using a paper-based system.
-
You also want an EDC solution that the clinics/sites can use easily. MedTech-specific EDC systems like Greenlight Guru Clinical employ an intuitive user interface that allows for quick onboarding and are easy to use without any prior programming experience or technical skills. Collaboration with the clinical teams at your study sites is paramount to the successful completion of a study, so a modern and user-friendly tool is essential.
-
It will also reduce the risk related to non-compliance, which could be a high cost to your business. For instance, EU MDR places much greater emphasis on clinical data for medical devices than the Medical Device Directives (MDD) did.
Creating this document should also help clarify the specific requirements you need from an EDC system, which will be invaluable during the vendor evaluation process.
![identify-stakeholders-sponsors--eqms-guide]() 3. Identify internal stakeholders and sponsors
3. Identify internal stakeholders and sponsors
Even though this is our third step, you should really begin identifying stakeholders as soon as you decide to build the case for a new EDC solution. Even if you’re spearheading the process, you need everyone who has a stake in this process to be involved.
Make a list of the key people who will need to be kept abreast of what’s happening or will be actively involved in carrying out this process.
There are a number of ways to structure a list of personnel, but a tool like a RACI chart (Responsible, Accountable, Consulted, Informed) may be useful to help you keep track of everyone and ensure you’re communicating well throughout the process.
Apart from the stakeholders that need to be involved in the buying process, the right EDC system enables you to collect high quality data that benefits the entire organization.
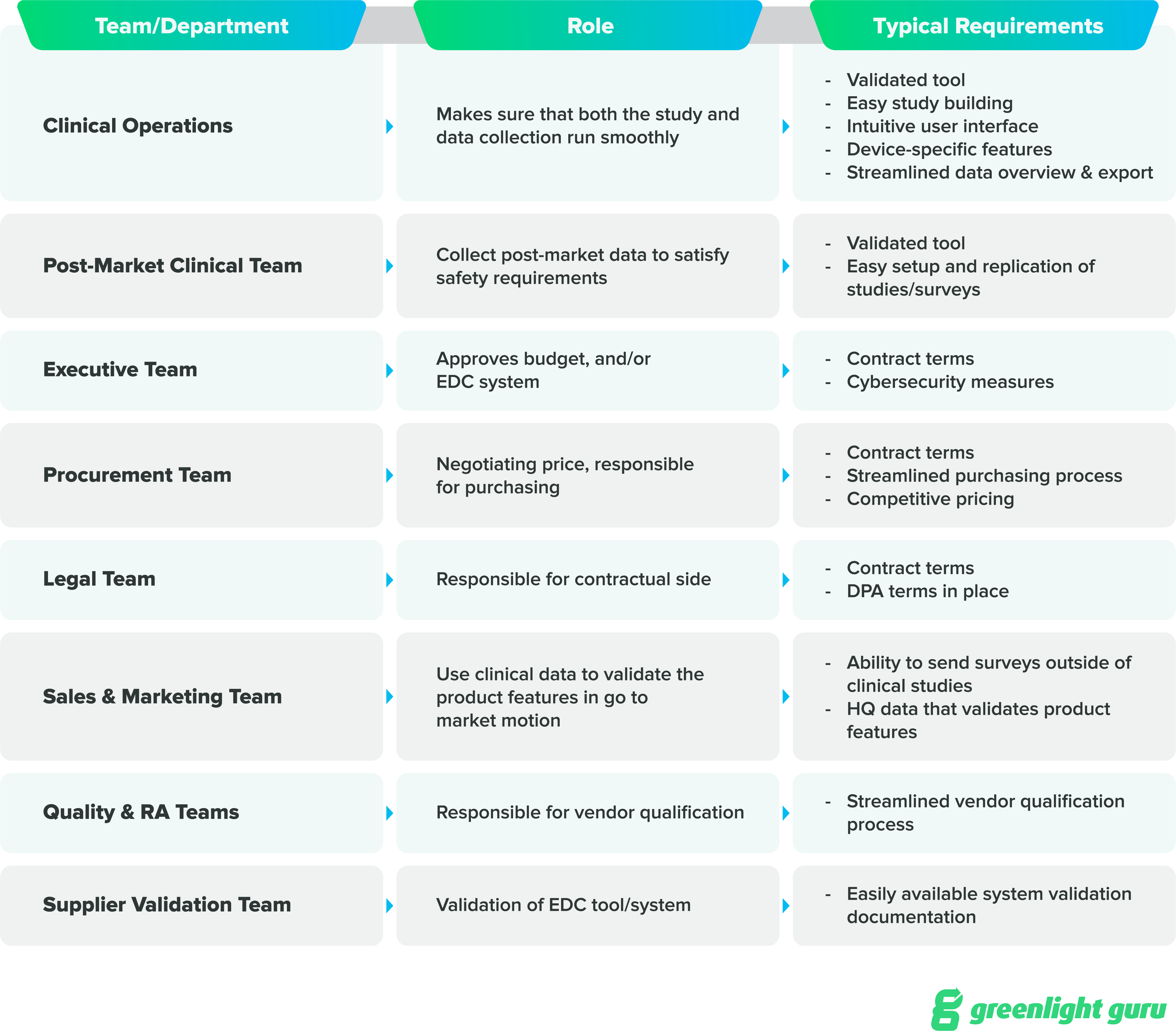
And once you’ve identified these stakeholders, be sure to get their opinions on the current EDC system and what they’d like to see in a new one. You may get some invaluable information on the team’s needs or hear a powerful anecdote that you can use when you’re presenting your business case to your exec team.
![evaluate-vendors]() 4. Evaluate vendors
4. Evaluate vendors
There’s a good chance your company will require you to formally evaluate multiple vendors and get more than one quote. Even if you feel strongly about a particular EDC solution, you’ll still need to go through a vendor evaluation process as you build your business case.
Think of this less an extra hoop to jump through and more of an opportunity to clearly distinguish a preferred solution from its competitors. Use the organizational challenges and opportunities that you previously documented to identify the solutions that will help you overcome those challenges and meet your objectives.
As you go through this process, a vendor evaluation checklist can be an excellent tool to keep track of the different options and quickly compare them. Here are 12 key questions you should be asking every EDC vendor:
-
Is this EDC system built specifically for MedTech companies?
-
How will your solution support our clinical data collection workflow?
-
Does your EDC software offer ISO 14155:2020 compliant safety reporting?
-
What is the workflow for documenting serious adverse device effects (SADEs)
-
Is your EDC software fully validated to ensure it functions as advertised?
-
How much work is required from the Sponsor to perform software validation?
-
Does your EDC facilitate out-of-the-box compliance with ICH Good Clinical Practice, the FDA’s 21 CFR Part 11, ISO 14155:2020, GDPR, HIPAA, etc.?
-
Do you have medical device experts on your customer success team? If not, what is your experience with MedTech regulations and requirements?
-
How quickly can this EDC solution be implemented?
-
How does your platform facilitate data privacy?
-
Do you have any metrics on satisfaction with your customer service? (Ex. Is there a cost related to tickets? How fast is the average ticket handled?)
-
Which statistical tools and data export formats does your product support?
-
What is the total cost of ownership (TCO) for your product?
Now, you may decide to outsource your clinical investigation to a CRO or consultant. But you should still ask questions about their preferred EDC solution. Before choosing a CRO, you might ask them similar questions like:
- Do you have a MedTech specific EDC?
- What is your track record with the current EDC?
- Should we decide to switch CROs in the future, are we able to take the EDC with us (take over the contract)?
- Can we contract directly with your EDC vendor?
- What are the timelines for a study build in your preferred EDC?
It’s also a good practice to speak with your industry peers who are using the different solutions to get their points of view. However, once you’ve found the vendor that meets your requirements and organizational needs, you need to be able to make the case for that specific EDC solution.
![build-the-case]() 5. Build the case for the solution you want
5. Build the case for the solution you want
If you have a specific vendor that you believe is the best fit for your company, talk to their sales team. They should be able to work with you to build a custom business case for their solution that takes into account your company’s unique circumstances.
As you start to build the case together, it’s important to include both the benefits of the EDC system you’d like to purchase, as well as the ways it will affect the costs of running clinical investigations and collecting data—in both obvious and less apparent ways. Cost-savings that might not be immediately recognized can include:
-
Less travel time or no travel at all (with data monitoring and real-time data from studies).
-
A reduction in time spent on data cleaning, data management, and prepping the data for analysis.
-
Shorter study timelines due to faster study setup and reduced study closure time.
In fact, G2 recently gave Greenlight Guru the “Best ROI” award among QMS software solutions.
As you work with the sales team, ask if they have any examples of customers who have experienced these kinds of cost-savings with their product. For instance, Oticon Medical estimates they now complete their clinical studies 130-140% faster since switching to Greenlight Guru Clinical. The Oticon team also estimated that their ROI on the purchase of Greenlight Guru Clinical was 136% after the first two years—and more than 270% after four years.
Quantitative data like that, combined with the qualitative benefits of your chosen solution, will be critical to helping you build a strong business case that you can present with confidence to your executive team.
6. Establish your transition plan
There’s one final piece of the puzzle you’ll want to have in place before you present your business case: the transition plan.
Also referred to as a project plan, the transition plan is the summary of how you intend to get from A to B—meaning from paper or your old EDC system to the new one. It should answer questions like:
-
What are the steps involved in transitioning to the new EDC system?
-
What kind of support will you get from the EDC vendor on the implementation?
-
Who will need to be trained on the new software and how long will that take?
-
Are there any risks involved in transitioning and what are you doing to mitigate them?
-
What is the estimated timeline for the transition, and how does it interact with key dates like the start of a study or regulatory submissions?
-
What are the specific data collection needs for any upcoming studies?
As you put together your transition plan, lean on your vendor's sales team and help them understand your timeline for implementation and any important dates or milestones you have, like an upcoming study you want to use this EDC system for.
As with every robust system, there will be an onboarding process. Learn from the sales team how long that will take, and when it is best to begin if you need to start your study in the next couple of months. With Greenlight Guru Clinical, medical device companies can set up their study within a couple of weeks and even faster depending on size or complexity. In some cases, our Customer Success team can even help with building parts of your first study to speed this process up.
7. Create your business case and present it
At this point, you’ve likely accumulated a mountain of documentation for this purchase. But to effectively present your business case to an executive team, you’ll need to be concise and clear.
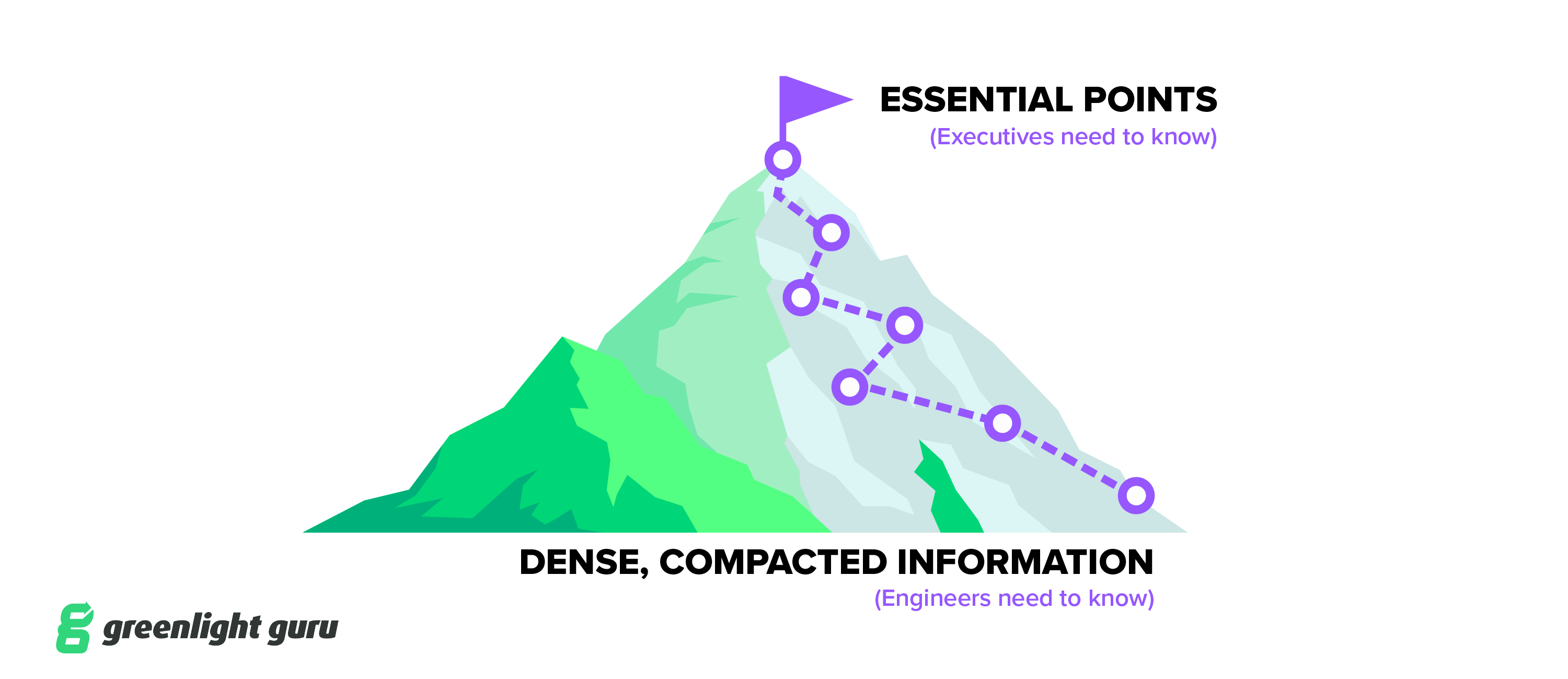
What your executives need to hear is the most important information—the peak of the mountain—what everything is building up to.
You don’t want to lead with the finer points of the adverse event module, for instance. You want to lead with the most important points to the business:
-
State the problem: Show why the current process does not work and illustrate the key challenges with the current EDC system or data collection tool.
-
Present the solution: Describe the specific benefits the new EDC system will provide to the company (ex. improved data accuracy and response rates, simplified regulatory compliance, faster study setup and completion, real-time data monitoring, shorter study timelines, etc.)
-
Vendor selection: Show how you evaluated the vendors and made the selection
-
Investment required: Outline the total cost of the solution
-
Long-term vision: Present the costs you’ll incur by doing nothing, and reiterate the benefits of the new system, especially the crucial impact on your clinical data and reduced time and money wasted
Turn that into an executive summary and a powerpoint that you can present, and be prepared to go into details if you’re asked questions about the solution. But if you’ve gone through the purchasing process step-by-step and done your due diligence ahead of time, you may just find that getting a ‘’yes’’ isn’t as difficult as you imagined.
Final thoughts![final-thoughts-eqms-guide]()
Purchasing a new EDC system is a big undertaking, but if you choose the right partner, it can have cascading positive effects on your business. The ability to quickly set up studies, get real-time visibility into clinical data, and improve response rates will lead to better clinical data, faster.
And when you choose a partner like Greenlight Guru Clinical, you’re not only getting the leading toolbox for MedTech clinical trial data collection, you’re getting dedicated customer service from MedTech professionals who understand the unique nature of medical device clinical trials.
So if you’re looking for a partner who will be with you every step of the way, then get your free demo of Greenlight Guru Clinical and learn how we can help you make the switch from your old EDC or paper to a purpose-built solution as smooth and efficient as possible.
Páll Jóhannesson, M.Sc. in Medical Market Access, was the founder and former CEO of Greenlight Guru Clinical (formerly SMART-TRIAL) and is currently the EVP of Europe at Greenlight Guru.
Read More
5 Dos and Don'ts when Choosing a QMS Solution for Your Medical Device Company
Best QMS Software: Ultimate Guide to Comparing Quality Management System Solutions
11 Questions to Ask QMS Software Vendors in the Medical Device Industry
Get your free eBook PDF
Electronic Data Capture (EDC) Buyer’s Guide: Making the Business Case for a New EDC System








 2. Document your organization’s needs
2. Document your organization’s needs 3. Identify internal stakeholders and sponsors
3. Identify internal stakeholders and sponsors 4. Evaluate vendors
4. Evaluate vendors 5. Build the case for the solution you want
5. Build the case for the solution you want






