Explore All Guru Services
Partner with our Gurus who help you efficiently navigate medical device regulations, reduce risk, and prepare for successful launch and audits.

Get Strategic Guru Advising
Gain clarity around regulatory requirements and leverage industry best practices to achieve your goals faster.
Benefits:
- Meet regularly in focused 1-hour sessions
- Customized plan designed to meet your needs
- Support mission-critical processes
- Library of SOPs with regular updates

Get Real-Time Support
Save time with an industry sounding board on best practices right when you need it, and expedited software support.
Benefits:
- On-demand assistance and coaching
- Easy, in-app functionality
- Expedited software support
- Library of SOPs with regular updates

Strategize With A Guru On Specific Topics
Strategic Guru session topics include:
- Quality system design recommendations
- Quality documentation review
- Design control best practices
- DHF / Technical File evaluation
- Risk management structures
- Product submission review
- CAPA & Complaint definition
- Regulatory strategy and planning
- Quality event support
- Audit preparation and practice

Get Strategic Guru Advising
Gain clarity around regulatory requirements and leverage industry best practices to achieve your goals faster.
Benefits:
- Meet regularly in focused 1-hour sessions
- Customized plan designed to meet your needs
- Support mission-critical processes
- Library of SOPs with regular updates

Get Real-Time Support
Save time with an industry sounding board on best practices right when you need it, and expedited software support.
Benefits:
- On-demand assistance and coaching
- Easy, in-app functionality
- Expedited software support
- Library of SOPs with regular updates

Strategize With A Guru On Specific Topics
Strategic Guru session topics include:
- Quality system design recommendations
- Quality documentation review
- Design control best practices
- DHF / Technical File evaluation
- Risk management structures
- Product submission review
- CAPA & Complaint definition
- Regulatory strategy and planning
- Quality event support
- Audit preparation and practice
We Bring Deep Expertise in the MedTech and Medical Device Industry
500 +
years of industry experience300 +
companies in our partner network16
quarters rated a leader in G2 Medical QMS97 %
users ranked our quality of support at 97%How We Set You Up for Success
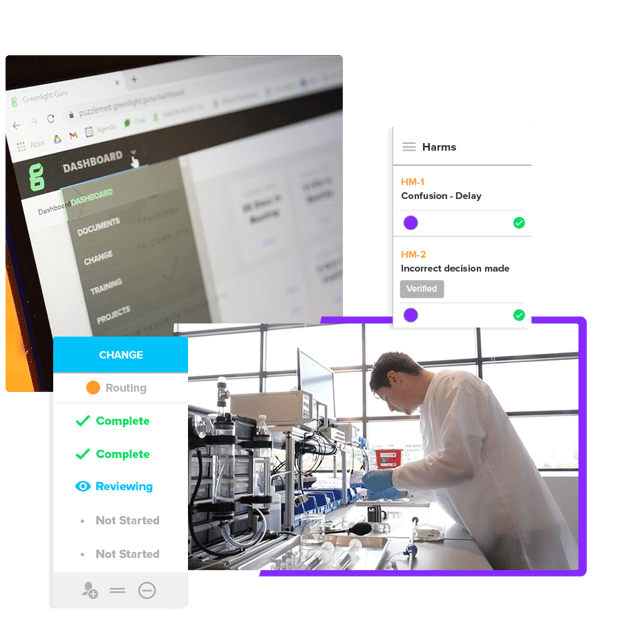
Streamline the Setup of Your Quality Management System
We've created 70+ audit-tested procedures, work instructions, and forms to save you time during implementation and put your team at ease during audits.
Our audit-tested QMS templates include:
- Quality Manual
- Management Review Template
- Project and Risk Plans
- Clinical Evaluation Templates
- Quality Event Procedures and Templates
- Supplier Survey Forms












