Greenlight Guru provides medical device companies with industry-leading software to bring life-changing products to patients faster, more efficiently, and with less risk.
You're Almost There...
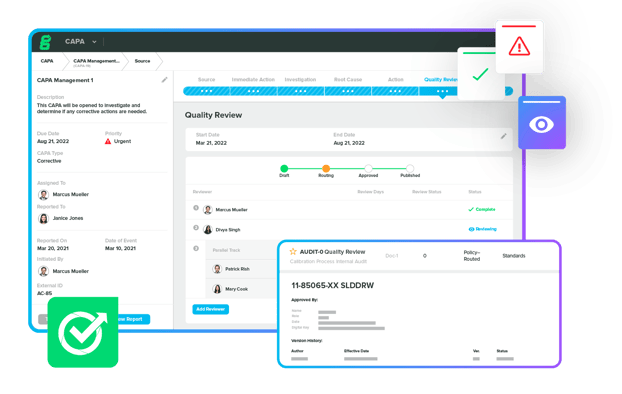
Rethink What Your QMS Can Do
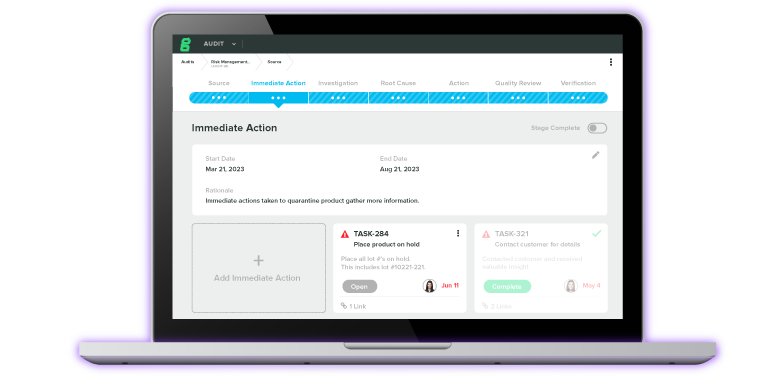
Upgrade your paper-based or generic QMS to a modern, cloud-based solution that enables you to ensure compliance, simplify audit prep, track quality events, and more.
Our QMS software helps you establish a single source of truth while scaling quality throughout your business.
Explore Greenlight Guru Quality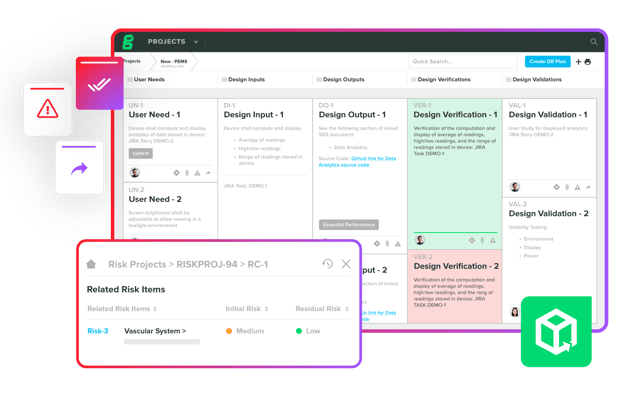
Bring New Innovations to Market Faster
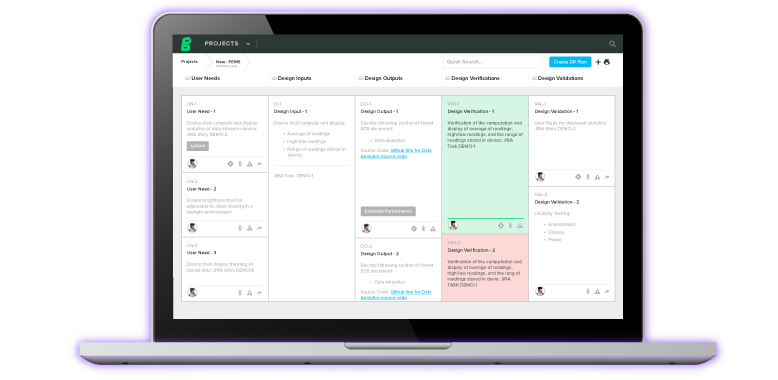
Streamline device documentation, accurately assess risk, and achieve traceability while you iterate through design and development.
Leverage collaborative workflows and automated compliance to accelerate timelines with greater efficiency.
Discover Greenlight Guru Product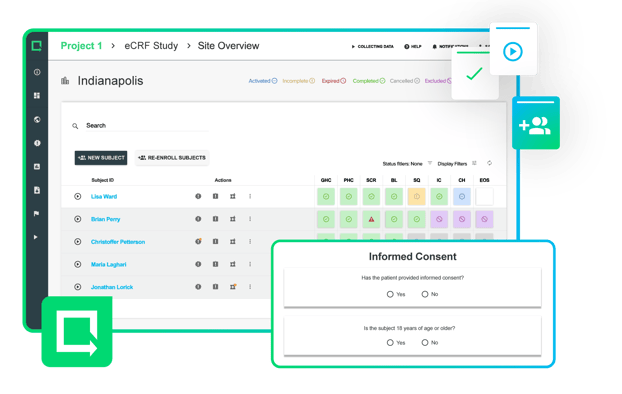
A Smarter Way to Collect and Manage Clinical Data
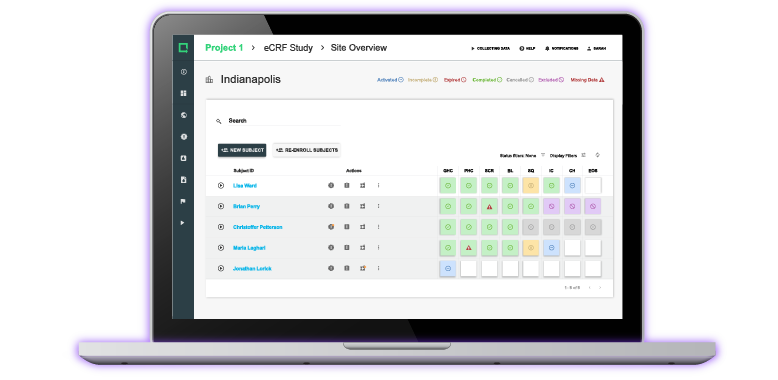
Collect and manage all clinical evidence, safety, and performance data within one versatile digital toolbox.
Modernize your process to cut weeks out of the clinical testing process and bring safer products to market.
See Greenlight Guru Clinicalfor Audits
Hands-on Guidance from Experienced MedTech Professionals
Greenlight Guru offers much more than software. Benefit from 1:1 human support covering regulations, risk reduction, software guidance, and business roadblocks.
Harness our 500+ years of combined industry experience to confidently meet your objectives at any stage of your journey.
Explore Guru ServicesOur Customers Say It Best
Greenlight Guru has been instrumental in helping us efficiently navigate the Quality Management System process and develop our FDA Submission.
Greenlight Guru has differentiated us from other start-ups. Our auditors told us, "Wow, you're steps ahead of anyone else."
Greenlight Guru Clinical (formerly SMART-TRIAL) helps us do better tests and faster tests. It will open up new opportunities in the way we do testing at Oticon.
Greenlight Guru has been instrumental in helping us efficiently navigate the Quality Management System process and develop our FDA Submission.
Greenlight Guru has differentiated us from other start-ups. Our auditors told us, "Wow, you're steps ahead of anyone else."
Greenlight Guru Clinical (formerly SMART-TRIAL) helps us do better tests and faster tests. It will open up new opportunities in the way we do testing at Oticon.

















_Leader_Mid-Market_EMEA_Leader.png)

_Leader_Americas_Leader.png)
_BestRelationship_Total.png)
_Leader_Europe_Leader.png)


