QMS Software
Ensure Compliance.
Lower Risk.
Accelerate Your Timelines.
Unlock efficiency with the leading cloud-based QMS software designed to improve speed and quality while lowering risk and cost.
 VIEW PRODUCT TOUR
VIEW PRODUCT TOUR
set up a QMS
for audits
audit findings
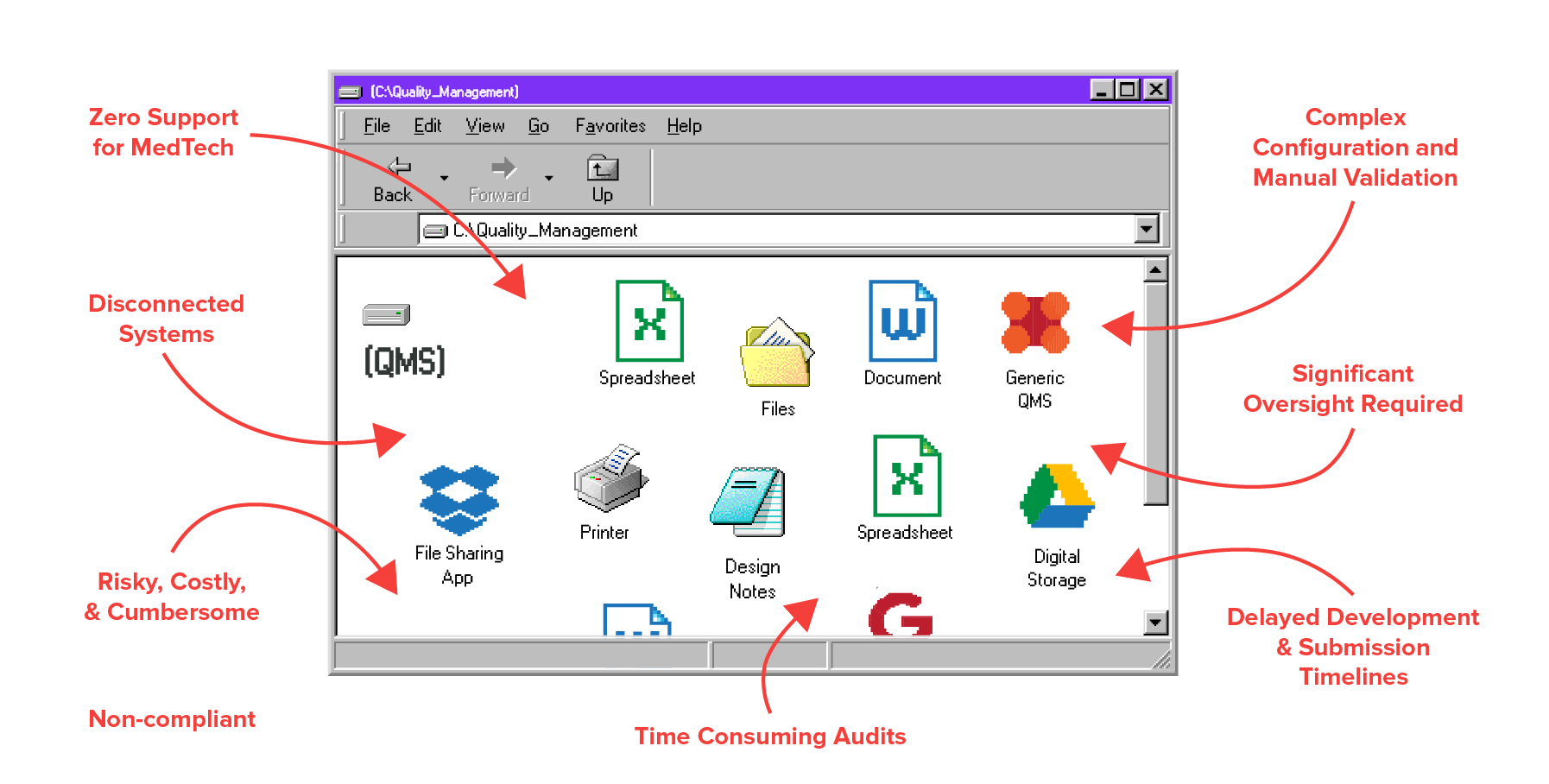
Built To Support Medical Device Companies
Your QMS software is optimized for the unique challenges and strict requirements of MedTech. Connect your people, processes, and data under one single source of truth.
Ensure compliance from the start without lifting a finger. Your software comes aligned with 21 CFR Part 820, ISO 14971:2019, ISO 13485:2016, and FDA requirements.
Enhance team collaboration, break down information silos, and maintain a single source of truth. By reducing risk of noncompliance, your device can stay on the market longer.
Create a culture of quality from idea, to development, to post-market surveillance under one connected system.
Spend less time digging through spreadsheets and binders with every auditor request. With one interconnected, living database, you can get through audits with fewer issues in just a few clicks.
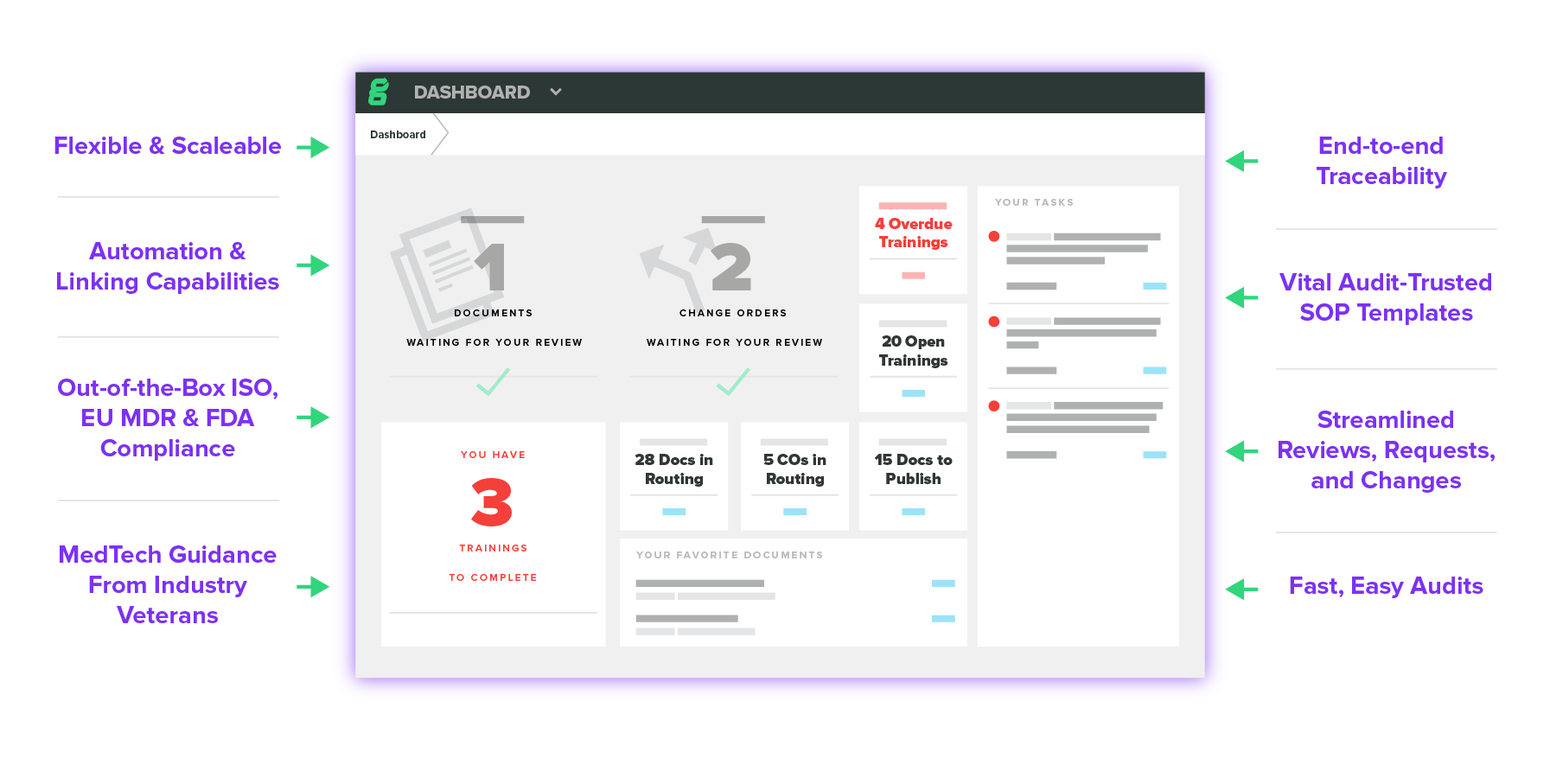
One Interconnected Quality Ecosystem
Unify your whole team under one easy-to-use quality management system.
Your documents should increase efficiency, not slow you down. Seamlessly review, approve, update, and link documents all in the cloud. All with a click.
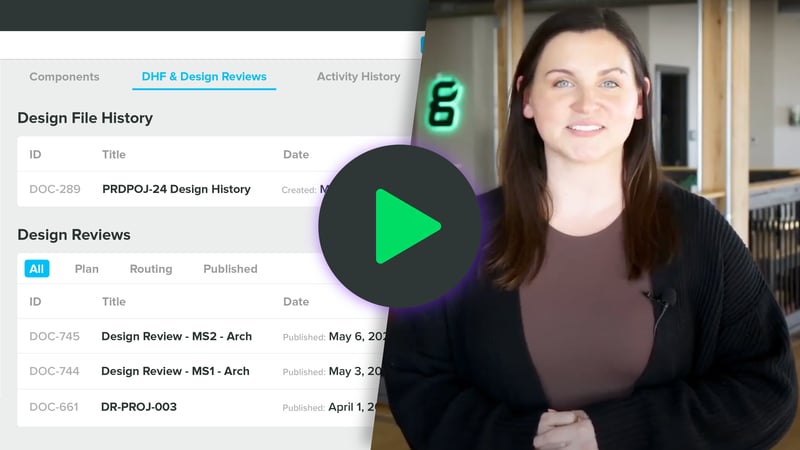
Learn MoreThe most advanced design controls functionality for MedTech. Intuitively integrate risk while you design, manage traceability, and maintain a living history file.
Learn MoreConnect your quality and product development teams to streamline collaboration, increase efficiency, and reduce risk across your product lifecycle
Learn MoreGive your team the tools they need to enable a culture of quality: compliant, yet flexible workflows for CAPAs, customer feedback, and audits.
Your company's success and your team's skills go hand in hand. Create, manage, and track individual or role-based training conveniently inside your QMS.
Learn MoreGet over 70 audit-tested and customizable templates. Streamline system set-up, ensure compliance, and follow industry best practices from the start.