Is Your QMS Stuck in the Past?
Nearly 50% of MedTech businesses are stuck with inefficiencies and outdated processes due to their paper-based or generic QMS.
Get A Live DemoThe Ugly Truth of a Paper-Based or Generic QMS
As technology rapidly changes how medical device companies operate, it’s clear that these outdated solutions simply don't work and hurt your bottom line.
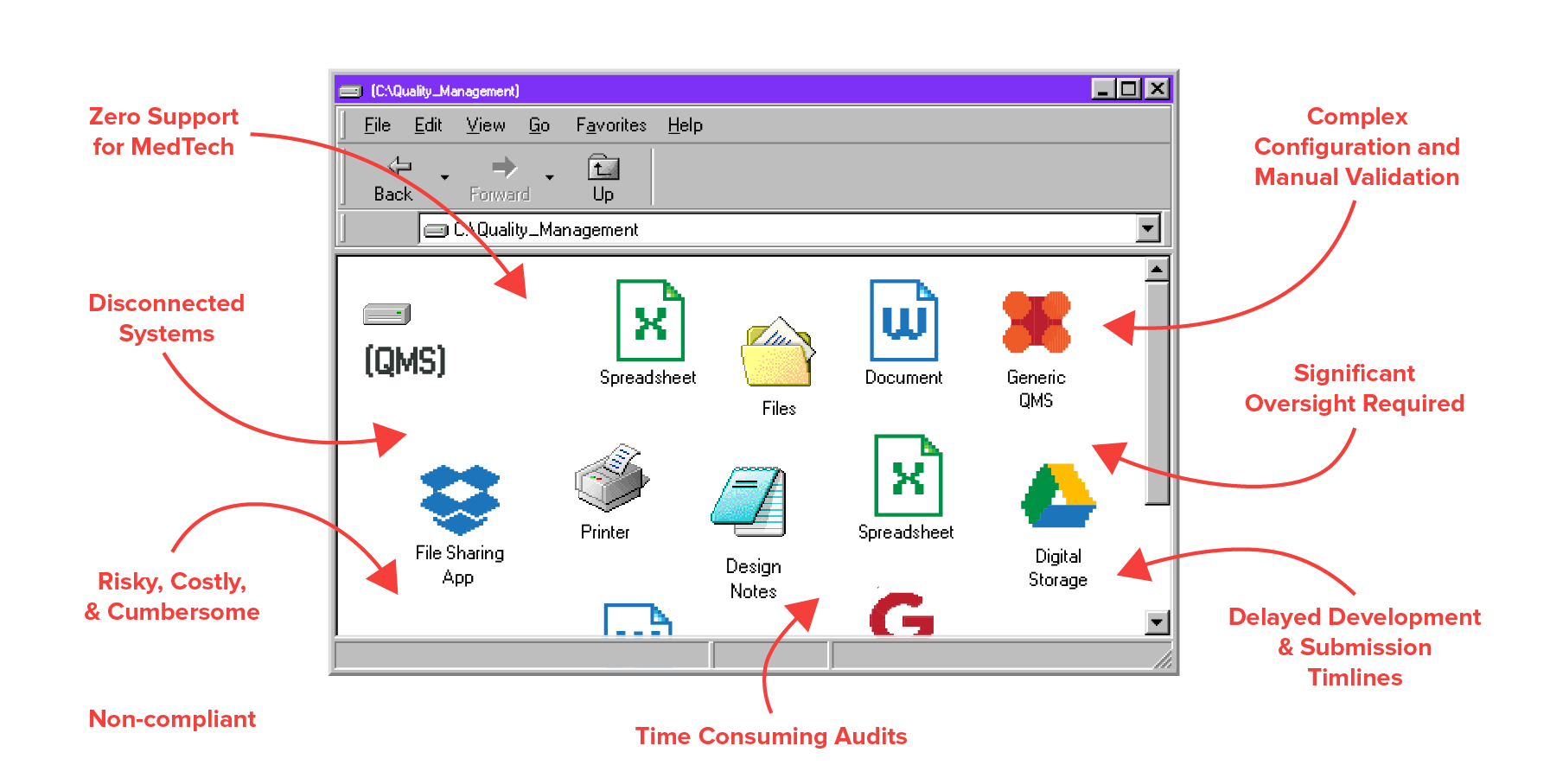
Zero Traceability
Lack of Efficiency
Risk of Poor Quality
It's Time to Upgrade to Greenlight Guru Quality
Specifically designed for MedTech companies, Greenlight Guru’s eQMS offers a modern solution to streamline design and development, connect your teams, and improve efficiency.
Your Old System
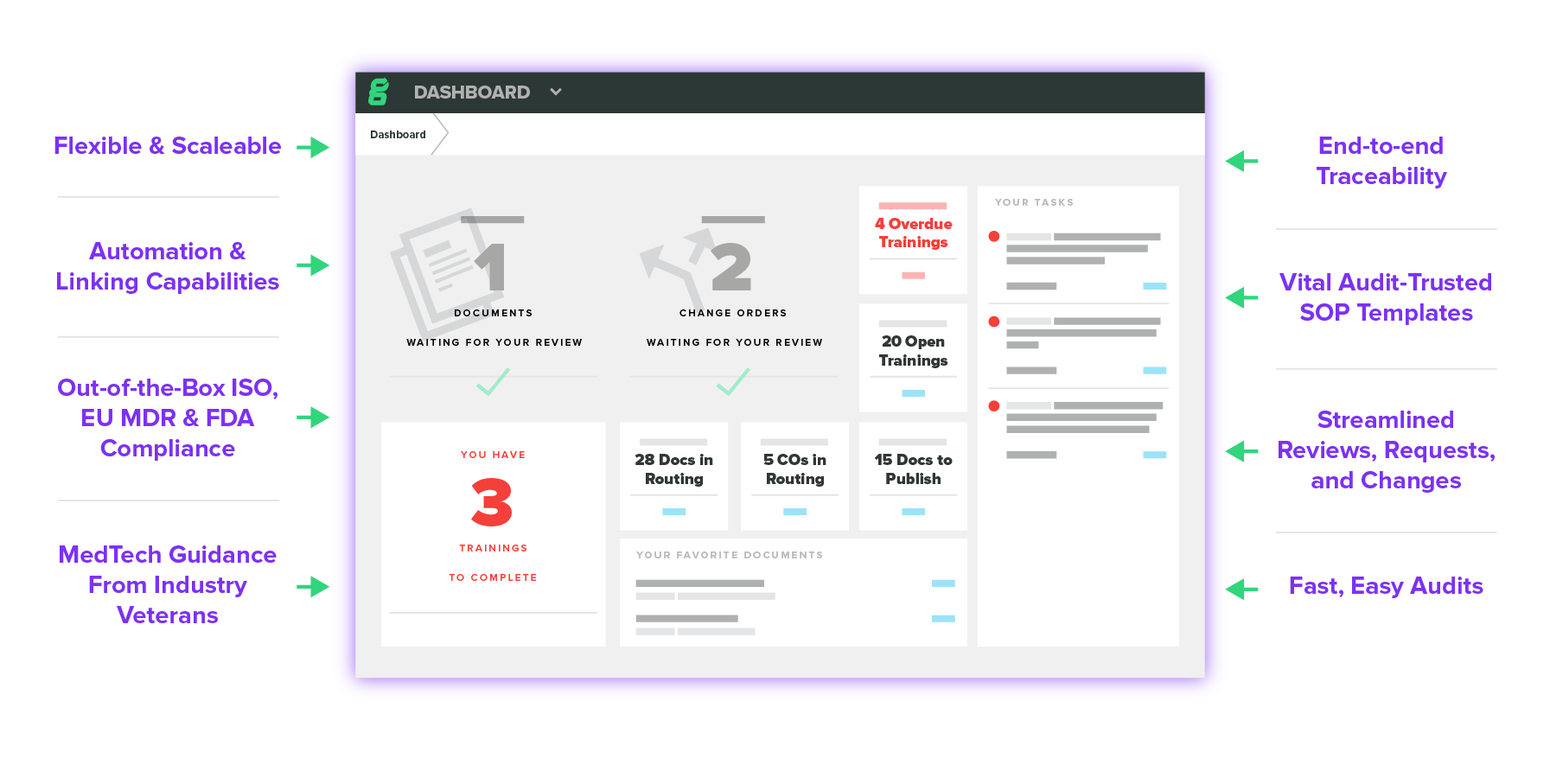
Greenlight Guru Quality
The Benefits of Switching to a Modern QMS
Leave outdated methods behind, and embrace the modernization of a purpose-built eQMS to move your MedTech business forward. Streamline processes while minimizing costly errors so you can bring higher quality and safer devices to market faster.
Always Audit Ready
Compliance Made Easy
Improved Efficiency
Built for Customers Like You
We're proud to work with over 1,100 of the best MedTech companies in the industry. Don't just take it from us. Discover what our customers have to say about us.

















.png)

.png)
-2.png)
.png)



