
We Are a Partner You Can Rely On
Don’t just take our word for it. Based on real customer feedback, high customer satisfaction scores, and large market presence, Greenlight Guru consistently ranks as a leader in both the Quality Management System (QMS) and Medical Device QMS categories on G2.
See Why We're #1 On G2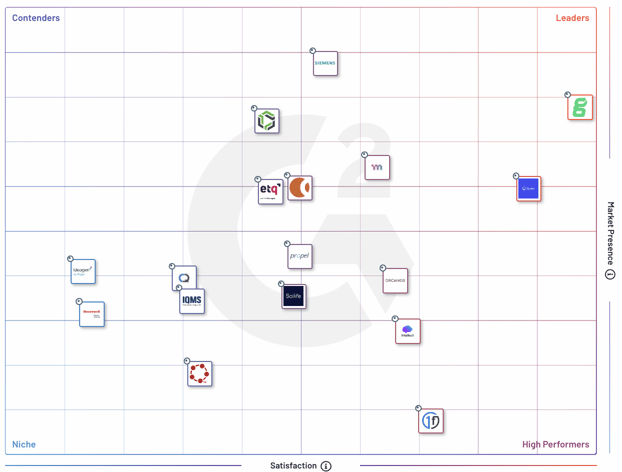
Trusted by Leading Medical Device Companies
According to G2's Spring 2024 Report, Greenlight Guru is yet again a quality management software Leader, based on high customer satisfaction scores and its large market presence.
For the nineteenth consecutive quarter, Greenlight Guru has been recognized as the Leader in Quality Management Software Solutions, as well as the #1 ranked solution for Medical QMS. Greenlight Guru also remains the chosen solution for QMS tools in Europe, ranking six points above the nearest competitor.
.png?width=622&name=Summer%2023%20G2%20Report%20(1).png)
The Only QMS Built for Medical Device Companies
We forgot to mention - we are so committed to medical device success that we made sure users had a place to seek out unbiased feedback, which is how the inception of G2’s Medical Device QMS category came to be in 2020.
Greenlight Guru has also maintained a Leader position in the Medical QMS software category for eight consecutive quarters with an overall satisfaction score of 98. As the only QMS exclusively built for medical device success, Greenlight Guru is proud to be trusted by leading, life-saving medical device companies globally.











