Medical Device Clinical Trials: Regulatory Pathways & Study Types Explained
.png?width=800&height=400&name=Medical%20Device%20Clinical%20Trials%20Regulatory%20Pathways%20%26%20Study%20Types%20Explained%20(1).png)
What is a clinical trial?
In both the US and the EU, medical devices may be required to undergo a clinical trial before they can be placed on the market. A clinical trial is a systematic assessment of the device’s safety and/or efficacy that uses human participants, and it’s a requirement for certain risk classes:
-
In the EU, all Class III and Class IIb implantable devices must undergo clinical investigations according to EU MDR.
-
In the US, all Class III devices are required by FDA to undergo clinical investigations as part of premarket approval (PMA).
With that in mind, let’s take a look at the different stages and designs of medical device clinical trials and the regulations surrounding them.
NOTE: You may see clinical trials referred to as “clinical studies” or, more commonly in the medical device industry, “clinical investigations.” These terms are all synonymous and can be used interchangeably.
How are medical device clinical trials initiated?
Because clinical trials involve human participants, medical device companies must perform preclinical testing and research on their product before even applying for a clinical trial.
Preclinical activities determine whether a device is safe and effective enough for use with human subjects, and include steps like:
-
Bench testing
-
Technical testing
-
Computer simulations
-
Animal studies
Once the manufacturer believes their device is ready for clinical trials, they must first get approval for their proposed investigation. The processes for getting approval and initiating a clinical trial in the EU and US are different, so let’s take a look at each.
Clinical trial regulatory pathways in the US
In the US, medical device manufacturers that want to pursue a clinical trial must obtain an Investigational Device Exemption (IDE). Only once the IDE has been approved can a device that has not yet received market approval be tested on human subjects.
There are exceptions to the IDE submission, which include certain low-risk diagnostic devices as well as devices that are determined to be non-significant risk (NSR).
If a device is granted an IDE, the clinical investigation must still be reviewed by an Institutional Review Board (IRB). Clinical trials are generally performed within an institution, such as a hospital, and an IRB is an additional layer of scrutiny that the institution provides to ensure the study meets its standards. The study may begin only once the IRB has approved it and FDA has approved the IDE application.
Clinical trial regulatory pathways in the EU
EU MDR has 20 articles outlining the requirements for clinical investigations of medical devices, spanning articles 62 through 82. Within these articles, the regulation lays out three regulatory pathways manufacturers can take:
-
Article 62 covers investigations that are performed in order to demonstrate conformity and obtain a CE marking. This is the pathway medical device companies will use if their device classification (forClass III or Class IIb implantables) requires a clinical investigation.
-
Article 74(1) covers the regulatory pathway for devices that already have a CE marking if the parameters of the investigation are within the device’s intended purpose. In other words, if you are conducting a clinical investigation as part of your Post-Market Clinical Follow-Up (PMCF), then you will be guided by Article 74(1).
-
Article 82 covers clinical investigations that are not being performed in order to demonstrate conformity. Additionally, the Member State in which you hold your study may have relevant national provisions for you to follow.
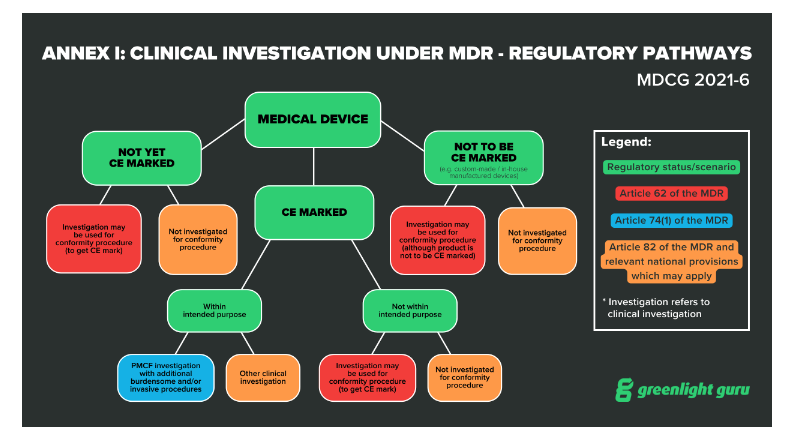 Annex I: Clinical Investigation under MDR - regulatory pathways, MDCG 2021-6 (Source)
Annex I: Clinical Investigation under MDR - regulatory pathways, MDCG 2021-6 (Source)
Before initiating a clinical trial in the EU, you’ll also need a CIV-ID and approval from the relevant competent authority. The CIV-ID is an EU specific tracking number that competent authorities in any Member State can use to identify and track your clinical investigation.
Keep in mind that once the EUDAMED database is fully functional, now scheduled for spring of 2024, the CIV-ID will be replaced by a Single Identification Number tracked through EUDAMED.
What are the different stages and types of medical device clinical trials?
Clinical trials may be carried out during both the premarket and postmarket phases of the device lifecycle. The graphic below includes the many different types of clinical activities, including clinical trials, medical device manufacturers may carry out during the pre-market and post-market phases.
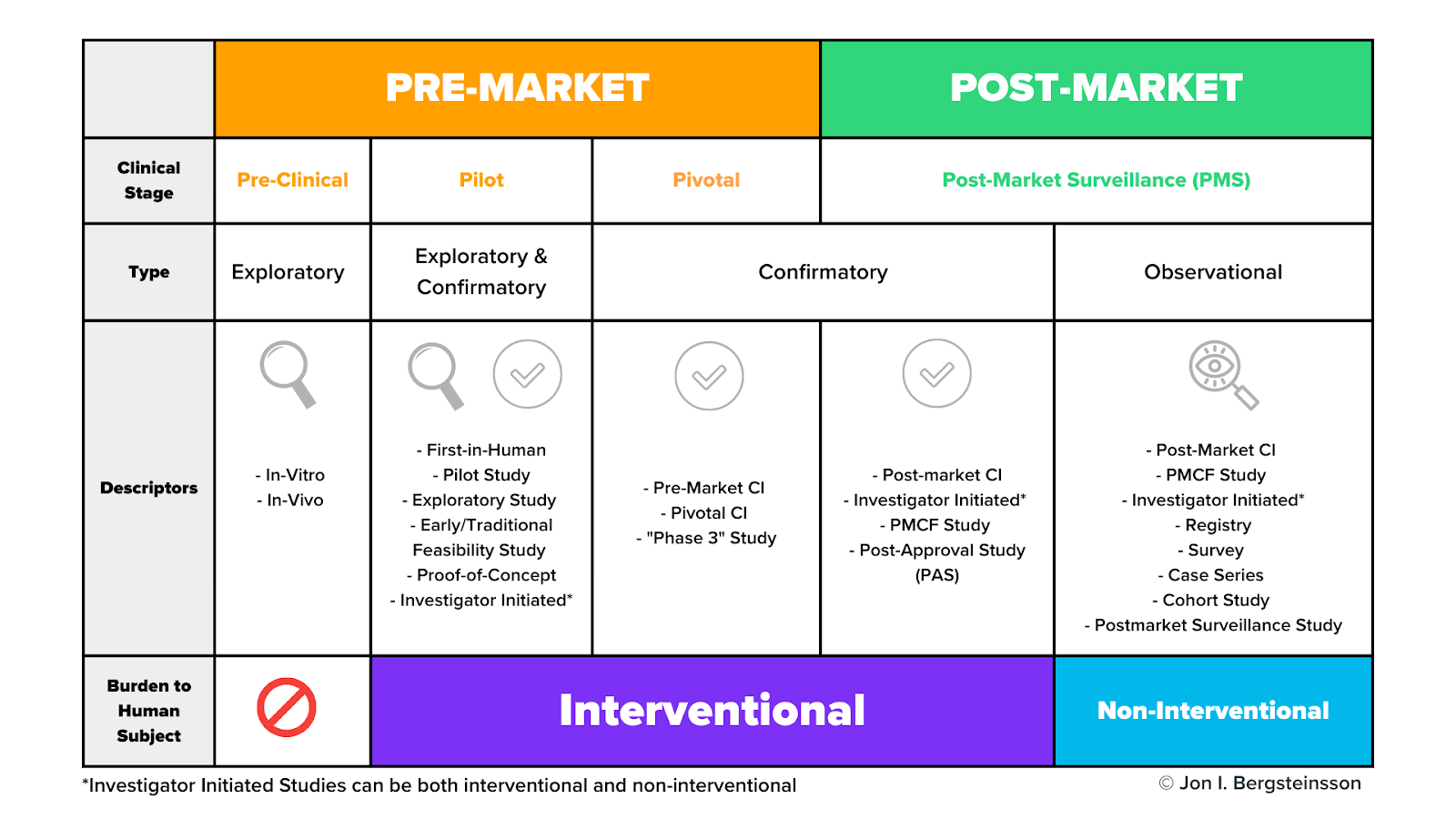
Clinical trials may occur during the pilot stage, the pivotal stage, or during post-market surveillance. As I mentioned earlier, pre-clinical activities do not use human subjects.
As we dig in here, don’t get too hung up on the study descriptors in this graphic. Many of these terms are interchangeable, and different descriptors are often used in different markets to describe the same thing. For now, let’s focus on the general types and stages of studies and their burden to human subjects.
What are pilot studies?
Pilot studies occur early in device development, often before the device design has been finalized. Pilot studies are used when nonclinical testing is unable to provide preliminary information on device functionality and clinical safety. These will be conducted with a very small number of patients—often 10 or fewer.
The purpose of pilot studies is to gain a broad range of information that may be used to:
-
Identify modifications to the device or procedure
-
Optimize operator technique
-
Refine the intended use population
-
Refine nonclinical test plans or methodologies
-
Develop subsequent clinical study protocols
The data you gain from a pilot study may then be used to help you design a pivotal study later on.
What are pivotal studies?
A pivotal study is used to gather definitive evidence of the safety and effectiveness of your medical device for a specific intended use. These studies generally use a larger number of subjects than pilot studies, and you’ll use the results of your pivotal study to gain regulatory approval for your device.
Keep in mind, a pivotal study does not necessarily need to be preceded by a pilot study. The types of clinical activities you carry out will depend on your device and the regulatory pathway you’re taking.
Do clinical trials happen during post-market surveillance?
As you can see from the graphic, the post-market surveillance stage includes both confirmatory and observational types of clinical activities.
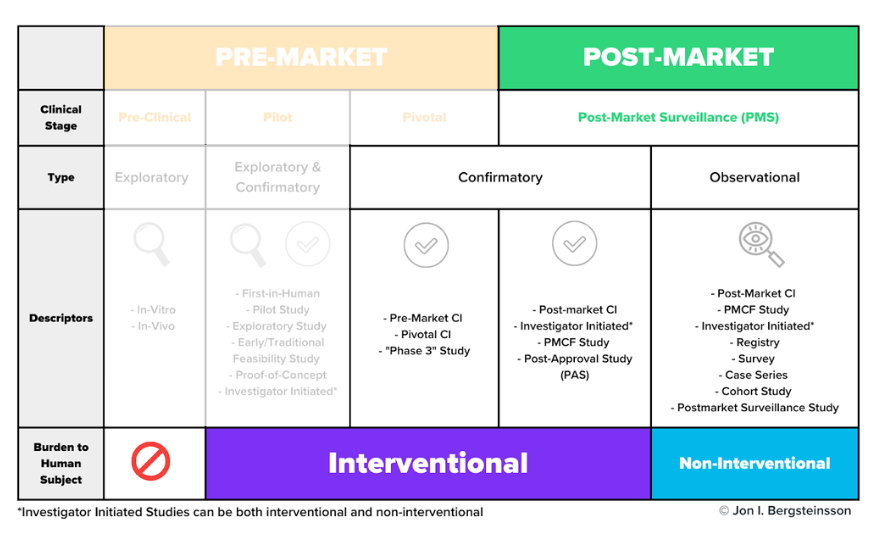
While it may seem odd that you would need to perform a confirmatory study after receiving approval to place your device on the market, this is not an irregular occurrence. For example, EU MDR includes a distinct regulatory pathway—Article 74(1)—for conducting a clinical investigation as part of your PMCF.
These post-market surveillance studies may be conducted for a number of reasons, including to confirm the safety and efficacy of the device once it’s on the market or to answer questions about the long-term safety or performance of the device.
How are observational clinical activities conducted?
Many post-market clinical activities are categorized as “observational” and they use non-interventional methods to collect data.
-
In interventional studies, such as a pivotal study, someone is actively recruiting participants. For example, a physician may ask a patient who may benefit from a certain device if they would like to volunteer for that study. In other words, they are intervening in the normal clinical pathway the patient would follow.
-
In non-interventional studies, there is no intervention in the clinical pathway—merely observation. For example, a physician prescribes a treatment they believe the patient needs (the normal clinical pathway), and then asks the patient if they would agree to share the data related to their treatment as part of an observational study.
Remember, some devices may need clinical data from all of these categories, but many will not. For example, low risk devices relying on well-known technology may not require any clinical investigations on your part.
Greenlight Guru Clinical helps you streamline clinical data collection for your medical device
This may seem like a complicated topic, but if you break it down by the stages of the device lifecycle and the type of clinical activity, you have a roadmap for how you’ll obtain the necessary clinical data for your device.
And when it comes time to begin collecting that data, you’ll need a flexible, modern platform that can streamline data collection from any and all of your clinical activities.
Greenlight Guru Clinical is that platform. Whether you’re gathering data in clinical studies, performance studies, PMCF/PMPF studies, surveys, registries, cohorts, or case series, our Electronic Data Capture solution allows you to collect and manage it all with ease. Even better, it comes fully validated out of the box per ISO 14155:2020.
Ready to learn more? Contact us today for a customized demo →
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.

.png?width=790&name=15-in-1%20clinical%20investigations%20content%20bundle%20(new).png)



