3 Factors that Impact Application of Decentralized Clinical Investigations for Medical Devices

The COVID-19 pandemic drove a huge interest in the concept of decentralized clinical investigations. The pandemic forced many MedTech manufacturers to re-evaluate their clinical operations, and even stop or delay ongoing clinical investigations.
But are decentralized trials the new way forward for manufacturers while at the same time complying with the new EU MDR on clinical evidence? To answer this question, we have to review the three main factors that impact application of a decentralized model for a medical device clinical investigation.
First, what is a decentralized clinical investigation?
Healthcare innovators and researchers have dreamt of offering remote treatment options using tele-technology for years. The aim is usually always the same, to reduce cost and improve clinical outcomes. But even in the countries with the most advanced healthcare systems, remote treatment options have still not fully replaced on-site hospital visits and face-to-face meetings with clinicians.
In recent years, the idea of applying the same methodology into clinical investigations has only gained more interest from sponsors, consultants and clinical service providers. Some eClinical service providers have started to promote the concept of virtual, remote, and decentralized clinical trials as a way to improve efficiency and reduce the cost of clinical operations.
While others are marketing it as the “Industry 4.0” upgrade in clinical research. However, when the COVID-19 pandemic hit us all, many service providers started promoting the concept as a business continuity solution. Using the pandemic as a stepping stone towards mainstreaming the ideology. In those cases the value propositions are mainly addressed at reducing on-site visits and keep studies running “remotely”.
But the concept of decentralized clinical investigations is not only about limiting on-site visits. Traditionally, clinical trials are usually centered around clinical sites that recruit and manage most subjects. The concept of decentralization is completely the opposite of that. Here, the patient is placed at the center of the investigation and not the clinical site or investigator.
The concept of decentralization is not an industry 4.0 buzz word. The concept is actually borrowed from political ideology, where it is all about distributing or delegating planning and decision making away from a central authority. But if one is to decentralize a clinical investigation, leaving planning and decision making in the hands of the patient might not be wise.
Instead, the concept is all about executing the study plan around the patient and his or her interests, and not the sites or sponsor alone. This includes e.g.
- Moving the study recruitment closer to patients by enabling more people to participate in relevant studies in their vicinity,
- Improving communication and data transparency towards the patient,
- Improving patient motivation and empowering participants to play a larger role in the study - in the hope of increasing adherence or compliance,
- Reducing the number of on-site visits to minimize interference with the patient’s day-to-day life, and minimize resource needs at clinical sites,
- Enabling patients to be in control of when (and sometimes how) to report clinical outcomes, e.g. from the comfort of their own home using their own devices,
- Increasing focus on patients’ participation, which is mirrored in high-quality support, communication and information sharing.
The study design of a decentralized clinical investigation tends to be based on remote consultations with data capture happening through virtual visits or electronic patient reported outcomes. But people often confuse decentralized investigations with fully remote study designs. To clarify, industry experts have categorized decentralized clinical investigations into two groups, i.e. hybrid and virtual or remote designs.
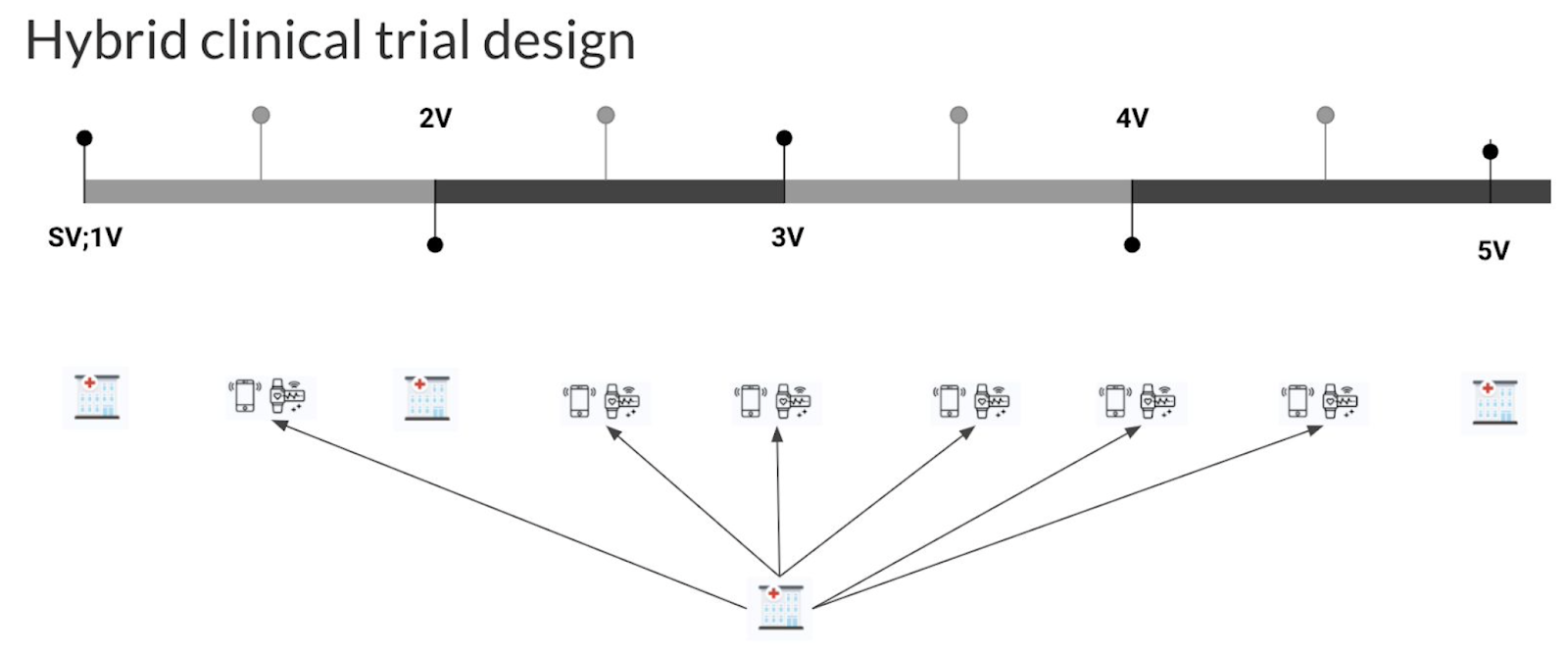 Image presented with permission from Studies & Me
Image presented with permission from Studies & Me
Hybrid study design includes at least one on-site visit while other activities are usually completed remotely, e.g. using smart devices and conference calls.
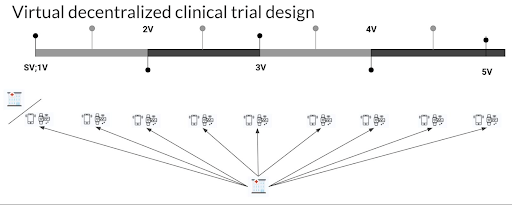 Image presented with permission from Studies & Me
Image presented with permission from Studies & Me
If the patient does not have to participate in any on-site visit and everything can be handled remotely, it’s categorized as a fully remote (or virtual) study design.
Three key factors that impact the applicability of a decentralized model for a medical device study
The new EU Medical Device Regulation is increasing the requirements for clinical evaluation and clinical data. In many cases, this forces companies to completely rethink their clinical strategy. As a result, manufacturers have to conduct more clinical investigations than ever before.
The increased focus on clinical data will greatly impact budgets and resources, especially for those who have never conducted clinical investigations before. It is therefore essential for those manufacturers who are initiating clinical investigations, that they do so efficiently and effectively.
But even though pharmaceutical sponsors might look at decentralized trials as the future, it might not be feasible for some medical device manufacturers.
This is primarily depending on the following three key factors:
1. Clinical feasibility and relevance for the device in question
While pharmaceutical trials are often similar in design, device studies can be as different as the devices are many. Pharmaceutical products can often be administered by the patient himself, as such, there are multiple cases where the application of a decentralized model is clinically feasible. But for many devices that’s not always the case.
There are multiple types of medical devices that are solely dependent on a highly skilled or trained clinical expert to be used in practice. As such, the application of a decentralized model might not always be likely to completely outweigh the traditional model. But in those cases where follow-ups can be conducted remotely, a hybrid model might be a possibility.
2. Size of the value driver (cost, compliance or data wise)
Taking all factors into account, the cost of conducting a decentralized clinical investigation might be higher than a traditional investigation. Thus, one must evaluate whether clinical outcomes, data quality, compliance/adherence, and other factors that might impact the result of the investigation outweigh possible changes in cost.
In that regard, taking into account the possibility of recruiting a potentially higher number of qualified subjects, higher data quality or better compliance, might impact the model to be chosen. Comparing the two models (decentralized vs traditional) based on cost alone, can be a very one sided judgment.
3. Cost of technology and service to support a decentralized design
95% of the MedTech industry in Europe are SMEs (MedTech Industry in Figures 2020). MedTech companies are often heavily focused on R&D and market access, which can result in regulatory and clinical teams often being left with limited resources to work with.
With the cost of decentralized trial technology (currently) being largely based on budgets for pharmaceutical companies, it can be difficult for the average MedTech company to acquire the necessary solutions and technology to implement a fully decentralized clinical investigation. As a result, some medical device manufacturers will have to compromise on some of the decentralized services, and implement a partly decentralized model. Somewhat of a hybrid-hybrid model.
Regulatory environment needs to change
MedTech companies that have devices that can benefit from a decentralized model, should evaluate the possibility. Not only because of the potential positive impact on recruitment, data quality or compliance, but because the service offering has greatly improved thanks to the COVID-19 pandemic.
But even though the industry might be showing more interest in the concept of decentralized clinical investigations, there are still regulations and local legislative environments that prohibit manufacturers in conducting a fully remote investigation.
For example, in some countries in Europe, informed consents need to be completed via on-site visits. On top of that, the standards for Good Clinical Practice, or other GxP standards, have not been amended to adhere to decentralized models. As such, local competent authorities find it difficult to fully accept decentralized clinical investigations.
Remote patient recruitment and data collection
With rapid improvement in eConsent technology, more secure electronic identification, and innovative and new ways to ensure GCP in a decentralized investigation, we might see this change for the better in the next few years. But whether legislators are ready to accept the change is a different question.
But one thing is clear, the life science industry needs to continue developing the model and the standards around it, and present guidelines and case studies in the hopes that legislators will start to listen and accept the model as a change for the better.
Since its inception, Greenlight Guru Clinical has been supporting medical device manufacturers in remote patient recruitment and data collection. Our Electronic Data Capture (EDC) solution comes pre-validated out-of-the-box and is the top choice for medical device companies.
Built to accommodate MedTech clinical operations and workflow, Greenlight Guru Clinical offers highly customizable eCRFs, bring-your-own-device ePRO to increase subject retention, and flexible survey options for clinical investigations or PMCF activities.
You are also welcome to contact us to get advice on the feasibility of a decentralized clinical investigation approach for your medical device.
Jón Ingi Bergsteinsson, M.Sc. in Biomedical Engineering, is the co-founder of Greenlight Guru Clinical (formerly SMART-TRIAL). He was also the technical founder of Greenlight Guru Clinical where he paved the way for the platform’s quality standards, data security, and compliance.





