If you think all QMS solutions are the same, let me introduce you to greenlight guru
Exclusively designed for medical device companies, Greenlight Guru is better than paper, smarter than Excel and SharePoint, and easier than legacy commercial EQMS Solutions.
The Greenlight Guru Platform
Designed for FDA 21 CFR Part 11 Compliance
With a system designed specifically to comply with Part 11, ensuring compliance becomes much simpler.
Intuitive To Use
Quality management software built for the 21st century.
Private Cloud-Based
Get more done with access to the system anytime, anywhere.
Secure
We don't take security lightly. Your data will be safe with us. Period.
Fanatical Human Support
A real person from our office will respond to you promptly - usually within an hour or two.
Scalable
Practical to use today and all the way through your growth phase past IPO.
Quality Management
Integrated Quality Processes
Manage all your quality processes in one closed loop, fully traceable system.
Real-Time Visibility
See in real-time the status of any and all processes.
User Dashboard
Highly configurable dashboards that may be personalized and shared across your organization.
Unlimited Projects & Users
Quality management systems must be accessible to everyone or it defeats the purpose.
Required FDA & ISO Documents
We give you the forms, templates & procedures you'll need to ensure your compliance.
Dynamic Reporting
Create, export, and share user defined reports.
Metrics
Gain better insight with accurate & complete metrics in one location.
e-Signatures
Sign off on processes, approvals, and reviews with electronic signatures.
Unified Management Dashboard
Get better visibility and control throughout the entire organization.
Live Workflow Status
Quickly check the status of any document revision or process.
Export Data
It's your data. You can export it if you need to.
Role Based Security
Make sure the right people, have access to the right data, at the right times.
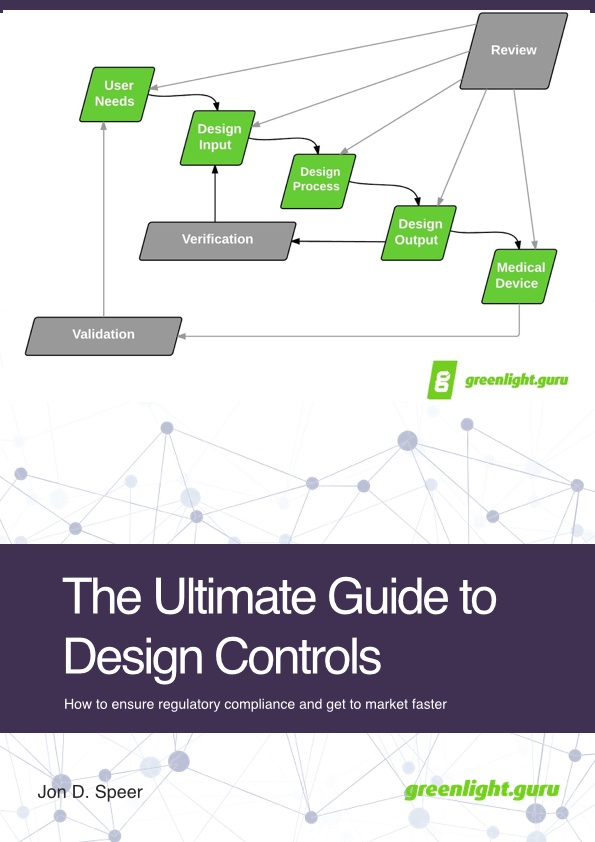
Design Control
FDA Best Practices
Simplify & ensure your compliance with a system designed to force FDA best practices.
Automatically Link Design Artifacts
Design artifacts are always visible and linked.
Design History Files (DHF)
DHF is always up-to-date and audit ready.
Design Reviews
Easily conduct design reviews electronically with everyone having access to the correct data.
Revision Control
Stay working on the correct document version all the time, every time.
Traceability Matrix
Easily create your trace matrix earlier in the process & have it always automatically updated.
Electronic Sign Offs
Use compliant e-Signatures for reviews and approvals.
Controlled Sharing
Manage who sees what projects and the level of detail they have access to.
Collaborative Workspace
Make sure the right people, have access to the right data, at the right times.
V&V Testing
Verify completed tests and approved or changed requirements electronically.
Manage Product Requirements
Keep your whole team up-to-date by managing all your product-related requirements in one place.
Complete Audit Trail
Always be able to quickly & easily provide objective evidence.
Analytics & Reporting
Perform better project, gap, and risk analysis with the metrics you need at your fingertips.
Email Notifications
Instant notifications about changes, updates, tasks & approvals.
Advanced Searching
Powerful search and quick retrieval of objective evidence and artifacts.
Risk Management
Aligned with ISO 14971
The flow and terminology we use in our software was designed to directly align with ISO 14971:2007 and EN ISO 14971:2012.
Link Design Controls & Risk Management
Link design and risk artifacts together directly in your traceability matrix with the press of a button.
Determine Harm
Determine harm that can result from each hazardous situation.
Link Risk-Benefit Analysis
Link the documentation you need to provide as objective evidence to support the medical benefits that outweigh a particular risk.
Risk Level Charts
Fully configurable risk level chart that allows you to select the severity / probability levels that make the most sense for your company.
Identify Hazards
Hazard categories come built in and are based on Annex E of ISO 14971.
Estimate and Evaluate Risks
Estimate severity of the harm and probability of occurrence of the harm. Identify sources from design controls to ensure user needs and design inputs address risks.
Automated Risk Evaluation
Once you estimate your risk level, we automatically evaluate the risk level based on the criteria you establish for risk acceptability.
Living Risk Management File
For every project created, a risk management file is created and automatically updated throughout the product’s lifecycle whenever a change is made.
Identify Hazardous Situations
Easily identify foreseeable events that can lead to a hazardous situation.
Identify Risk Controls
Ability to identify risk controls and evaluate risk after those risk controls. Link specific design controls as risk control measures.
Update Residual Risk
After you estimate and evaluate a risk for a hazardous situation, you are able to update the residual risk.
Document Management
Complete Audit Trails
Always be able to quickly & easily provide objective evidence.
Single Repository
One place to manage all your product & non-product related documentation.
Workflow Status
Easily check on the status of any document or process.
Change Control
Makes verifying change or approval requirements a snap.
Electronic Signatures
Automatically and securely authenticate users' electronic signatures.
Revision Control
Maintain compliance and simplify audit preparation with complete document revision control.
Instant Notifications
Everyone's notified about changes, tasks & approvals in real time.
Controlled Viewing, Printing & Exporting
Configure watermarking, overlays & other view and print prompts.
Automated Routing, Reviews, Escalations & Approvals
Improve your cycle times with process automation.
Multi-Dimensional Linking
Link design elements one-to-one, one-to-many or many-to-many.
Automatically Link Documents
Forces you to have traceability throughout the entire system & mitigates risk.
Advanced Search
Locate any document with advanced search capabilities
Bulk Uploads
Upload as many documents as you'd like at once using simple drag and drop functionality.
Retire & Archive Documents
Keep your system & data up to date and accurate.
Categorize, Classify, & Tag
Always be able to find any document or record instantly with advanced meta data tags.
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)