MDSAP ISO 13485:2016
FREE GAP ASSESSMENT TOOL
This multipurpose resource has built-in functionality to assess quality system requirements of the ISO 13485:2016 standard alongside the specific requirements of each auditing organization participating in MDSAP, allowing medical device companies to identify gaps within their QMS processes and track subsequent changes throughout implementation.
Features & Benefits
I want this free tool!
Features & Benefits

Get your free
MDSAP vs. ISO 13485:2016 Gap Analysis Tool

MDSAP
3 things you need to know about Medical Device Single Audit Program
#1
There are five regulatory authorities that participate in MDSAP
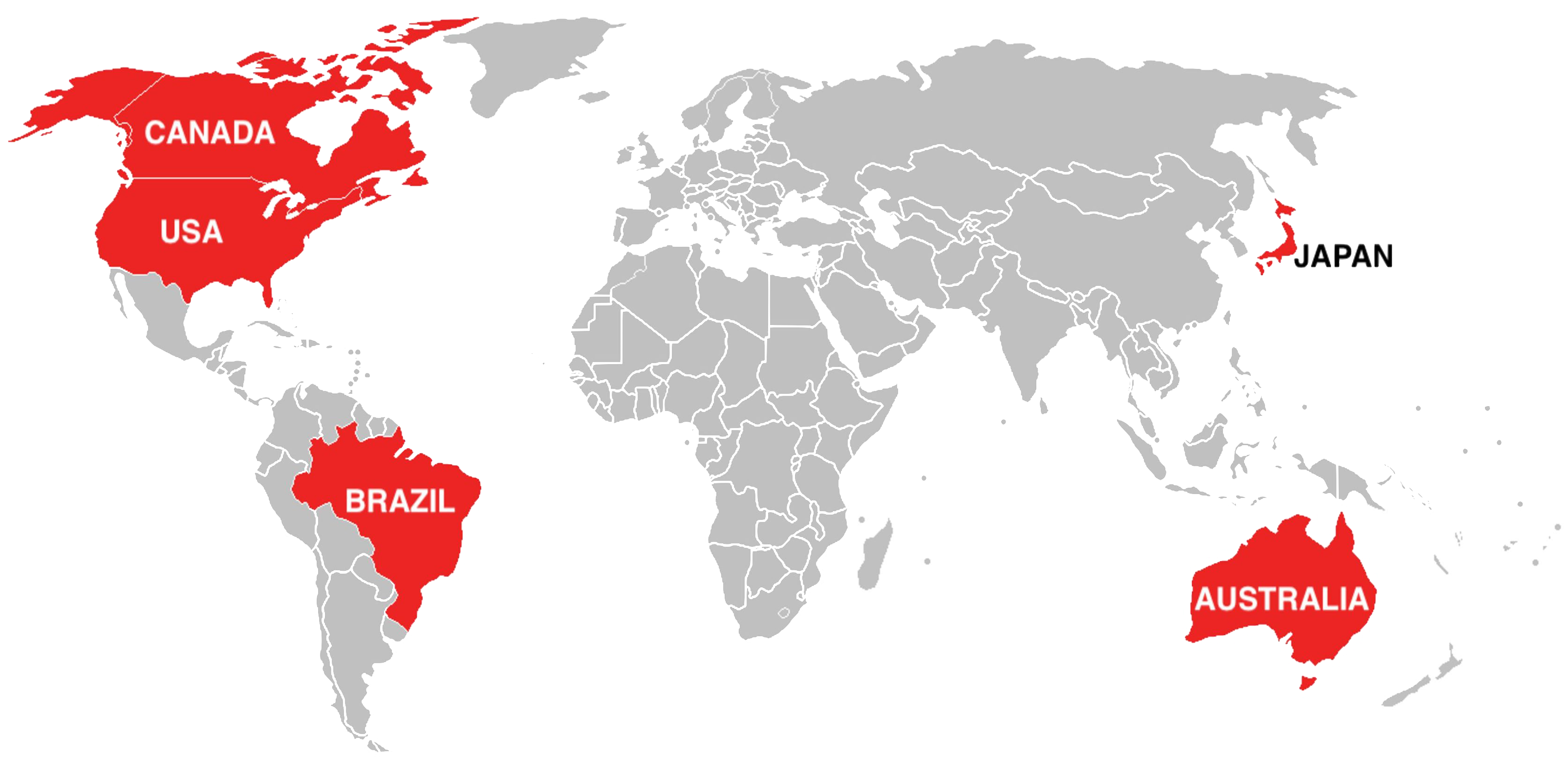
United States of America
Food and Drugs Administration (FDA)
Quality management requirements are based on 21 CFR Part 820.
Brazil
Agência Nacional de Vigilância Sanitária (ANVISA)
Quality management requirements are based on RDC ANVISA 16/2013.
 Japan
Japan
Ministry of Health Labor and Welfare & Pharmaceuticals and Medical Devices Agency (MHLW & PMDA)
Quality management requirements are based on MHLW Ordinance No. 169.
Canada
Health Canada
Quality management requirements are based on ISO 13485:2016.

Australia
Therapeutic Goods Administration (TGA)
Quality management requirements are based on TGA regulation (TG(MD)R Sch3).
#2
Audit Grading System for Nonconformities
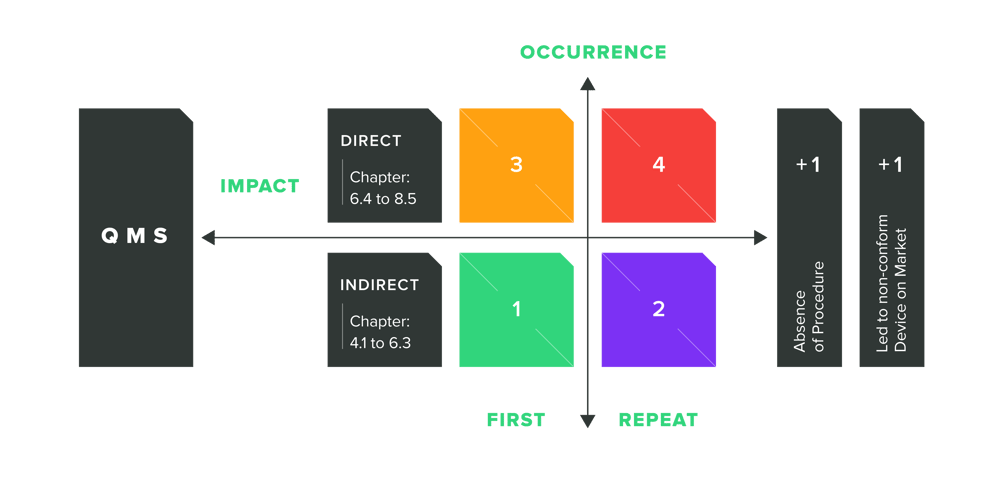
Direct nonconformities (Chapter 6.4 to 8.5) are the high-risk issues and starting at grading level 3 (first nonconformity) or grading level 4 (repeated nonconformity). Depends on the deviation, additional gradings can be put on top. For example, the deviation has an absence of procedure or it ends in a non-conformity product on the market (+1 Level each).
Indirect nonconformities (Chapter 4.1 to 6.3) are the low-risk issues and normally starting with grading level 1 (first nonconformity) or grading level 2 (repeated nonconformity). Depends on the deviation, additional gradings can be put on top. For example, the deviation has an absence of procedure or it ends in a non-conformity product on the market (+1 Level each).
If the Auditing Organization finds three or more 4 levels or one or more 5 levels, these are strong indicators that you will not pass the audit.
#3
90-days following an Audit
This timeline shows the next steps of both manufacturer and Auditing Organization after an audit ends.

Day 0-5: Auditing Organization notifies MDSAP Regulatory Authority in the case that, more than two Grade 4 or one or more Grade 5 nonconformities have been identified.
Day 0-15: Medical device manufacturer provides remediation plan for complying with quality system regulations.
Day 0-30: Manufacturer provides evidence of implementation of remediation actions that address the Grade 4 or Grade 5 nonconformities.
Day 0-45: Auditing Organization submits a complete audit report to Regulatory Authorities in the case of a 5-day notice.
Day 0-90: Auditing Organization submits a complete audit report to Regulatory Authorities in the event the 5-day notice criteria is not met.

ABOUT GREENLIGHT GURU

ABOUT Regulatory Globe
To develop medical products that improve quality of life and extend life expectancy is the goal of the medical device industry. In order to serve this basic need optimally, laws and regulations are inevitable. Such regulations ensure safety and quality of products as well as raise the state of the art and thus promote progress steadily. This is the reason why it is so important to take the new requirements as an opportunity for improvements and not as a burden. Only companies that recognize this will be successful in the long term.
This is where the task of Regulatory Globe begins. Together with our partners, we specialize in monitoring laws and regulations and provide tools that help our clients improve their products processes. This will eventually benefit the people in need of these medical devices. Visit our website to learn more by clicking here.
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)




