Economic Operators under EU MDR
FREE GUIDANCE DOCUMENT

What is included?
- Economic Operators EU- MDR 2017/745 Guidance Document
- Economic Operators under EU MDR
- Identification within the supply chain
- Economic Operators under EU MDD
- Comparison of Economic Operators under EU MDD and EU MDR
- Manufacturer
- Authorised representative
- Importers
- Economic Operators under EU MDR

50% Completed
Get your free
Economic Operators EU MDR Guidance Document Tool

Who are the economic operators?
Answers to your questions
ECONOMIC OPERATORS: WHAT IS IT AND WHY DOES IT MATTER?
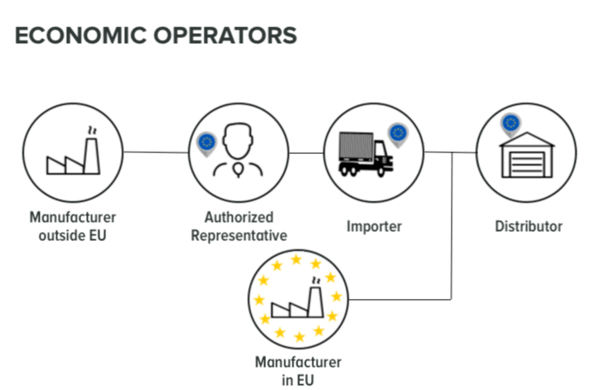
Economic Operators is a term not identified in the Medical Devices Directive 93/42 EC. Since the release of the new MDR EU 2017/745 Economic Operators have more responsibility. The EU MDR 2017/745 named the manufacturer, the authorised representative, the importer and the distributor as Economic Operators. Each economic operator has individual obligations, although all the economic operators have to collaborate together in order to make the device available on the market. The manufacturer must ensure that the supply chain - economic operators are aligned to the EU MDR.
Manufacturers shall establish, document, implement, maintain, keep up to date and continually improve a quality management system (QMS) to ensure compliance with EU MDR in the most effective manner and in a manner that is proportionate to the risk class and the type of device.
Why should I download this guidance document?
This document illustrates the relationship between the economic operators and addresses the responsibilities of each one. Through infographics and examples, this document aims to give the best guidance to the reader in order to become familiar with the economic operators adopted by EU MDR 2017/745.
Economic Operators is a term not identified in the Medical Devices Directive 93/42 EC. Since the release of the new MDR EU 2017/745 Economic Operators have more responsibility. The EU MDR 2017/745 named the manufacturer, the authorised representative, the importer and the distributor as Economic Operators. Each economic operator has individual obligations, although all the economic operators have to collaborate together in order to make the device available on the market. The manufacturer must ensure that the supply chain - economic operators are aligned to the EU MDR.
Manufacturers shall establish, document, implement, maintain, keep up to date and continually improve a quality management system (QMS) to ensure compliance with EU MDR in the most effective manner and in a manner that is proportionate to the risk class and the type of device.
Why should I download this guidance document?
This document illustrates the relationship between the economic operators and addresses the responsibilities of each one. Through infographics and examples, this document aims to give the best guidance to the reader in order to become familiar with the economic operators adopted by EU MDR 2017/745.
LIMITED NUMBER OF DOWNLOADS AVAILABLE.
Get yours before they're gone!
Economic Operators under EU MDR
Free Guidance Document
submit this form to get instant access to this free Content Giveaway!

Visit Pharmi Med Template Shop for additional resources and tools. All templates are developed with consultants who have over 20 years’ experience in the Medical Device industry and documentation that has been through successful audits. For further assistance with documentation or using these tools, please contact Pharmi Med at info@pharmi-med.com or +44 (0)7752 144409.
I want this free content!

ABOUT GREENLIGHT GURU
Greenlight Guru is a modern quality management software platform used by medical device companies in over 25 countries to bring new products to market faster while simplifying regulatory compliance and reducing risk. Being the only industry specific, cloud-based eQMS, Greenlight Guru is easier to implement, easier to use and simply fits medical device processes out-of-the-box allowing companies to innovate faster and be more efficient. Click here to learn more.

ABOUT Pharmi Med Ltd
Pharmi Med Ltd. is a rapidly growing regulatory and compliance consultancy which has served the Medical Device and Pharmaceutical manufacturing industries since 1999. Pharmi Med Ltd has a strong reputation of delivering on projects, ensuring satisfactory audits and patient safe products.
We offer solutions to manufacturers where budgets are restrained and permanent headcount is not the answer. With ever changing regulations, we take on the responsibility to stay informed and implement the requirements so that manufacturers can focus on production. We aim to ensure you have a competitive advantage by ensuring your products are meeting US FDA and EU requirements, as well as any other global market.
Pharmi Med Ltd is a member of Medilink and Bionow. We are also IRCA certified so that you can be rest assured your audits will be performed at a global standard.. Visit our website to learn more by clicking here.
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)

