Economic Operators under EU MDR: Free Guidance Document
What is included?
Economic Operators EU MDR 2017/745 Guidance Document
- Economic Operators under EU MDR
- Identification within the supply chain
- Economic Operators under EU MDD
- Comparison of Economic Operators under EU MDD and EU MDR
- Manufacturer
- Authorised representative
- Importers
Who are the Economic Operators?
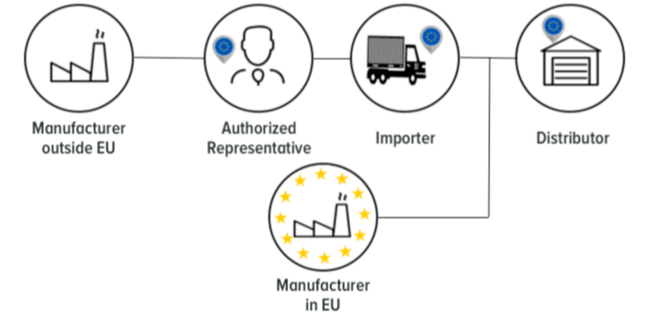
Why should you care about Economic Operators?
Economic Operators is a term not identified in the Medical Devices Directive 93/42 EC. Since the release of the new MDR EU 2017/745 Economic Operators have more responsibility. The EU MDR 2017/745 named the manufacturer, the authorised representative, the importer and the distributor as Economic Operators. Each economic operator has individual obligations, although all the economic operators have to collaborate together in order to make the device available on the market. The manufacturer must ensure that the supply chain - economic operators are aligned to the EU MDR.
Manufacturers shall establish, document, implement, maintain, keep up to date and continually improve a quality management system (QMS) to ensure compliance with EU MDR in the most effective manner and in a manner that is proportionate to the risk class and the type of device.
Why should you download this resource?
This document illustrates the relationship between the economic operators and addresses the responsibilities of each one. Through infographics and examples, this document aims to give the best guidance to the reader in order to become familiar with the economic operators adopted by EU MDR 2017/745.
Visit the Simplimedica Template Shop for additional resources and tools. All templates are developed with consultants who have over 20 years’ experience in the Medical Device industry and documentation that has been through successful audits. For further assistance with documentation or using these tools, please contact Simplimedica at info@simplimedica.com or +44 (0) 77 52 14 44 09.
I want this free content!

ABOUT GREENLIGHT GURU
Greenlight Guru is a modern quality management software platform used by medical device companies in over 25 countries to bring new products to market faster while simplifying regulatory compliance and reducing risk. Being the only industry specific, cloud-based eQMS, Greenlight Guru is easier to implement, easier to use and simply fits medical device processes out-of-the-box allowing companies to innovate faster and be more efficient. Click here to learn more.
.png?width=319&height=67&name=SIMPLIMEDICA-LOGO-WITH-TAGLINE%20(1).png)
ABOUT Simplimedica
Simplimedica, FKA Pharmi Med Ltd., is a consultancy firm that believes in long-term partnerships. We support short-term, medium-term, and long-term project work for small medium-sized businesses as well as large corporations. You may have a shortage in skill or wish to outsource some work that may be more productive. Simplimedica has experience in a number of Pharmaceutical and Medical Device companies.
Adnan Ashfaq is a Quality, Regulatory & Validation Specialist with almost 20 years of experience in the Medical Device, Biotech, and Pharmaceutical industries. Adnan founded Simplimedica in 2011 and has since supported Medical device companies around the labyrinth of regulations and assisting them in flying through successful audits. He has worked with start-up companies and multinationals to develop new products since 1999. With regulations tightening, especially in Europe, Simplimedica enables and simplifies the compliance conundrum to ensure manufacturers reach their desired market and stay in the market.
Visit their website to learn more.
-2.png?width=500&height=501&name=GG-LinkedIn-profile-pic-green-1%20(1)-2.png)

