
.png?width=2400&name=mdQMS%20chart%20(5).png)
EASY TO USE, FAST TO IMPLEMENT MEDICAL DEVICE QUALITY MANAGEMENT SOFTWARE THAT CONNECTS DISPARATE PROCESSES, SOURCES, PEOPLE, AND DATA FOR THE FIRST TIME EVER. INCREASED VISIBILITY SMOOTHS YOUR PATH TO COMPLIANCE AND LETS YOU FOCUS ON TRUE QUALITY.
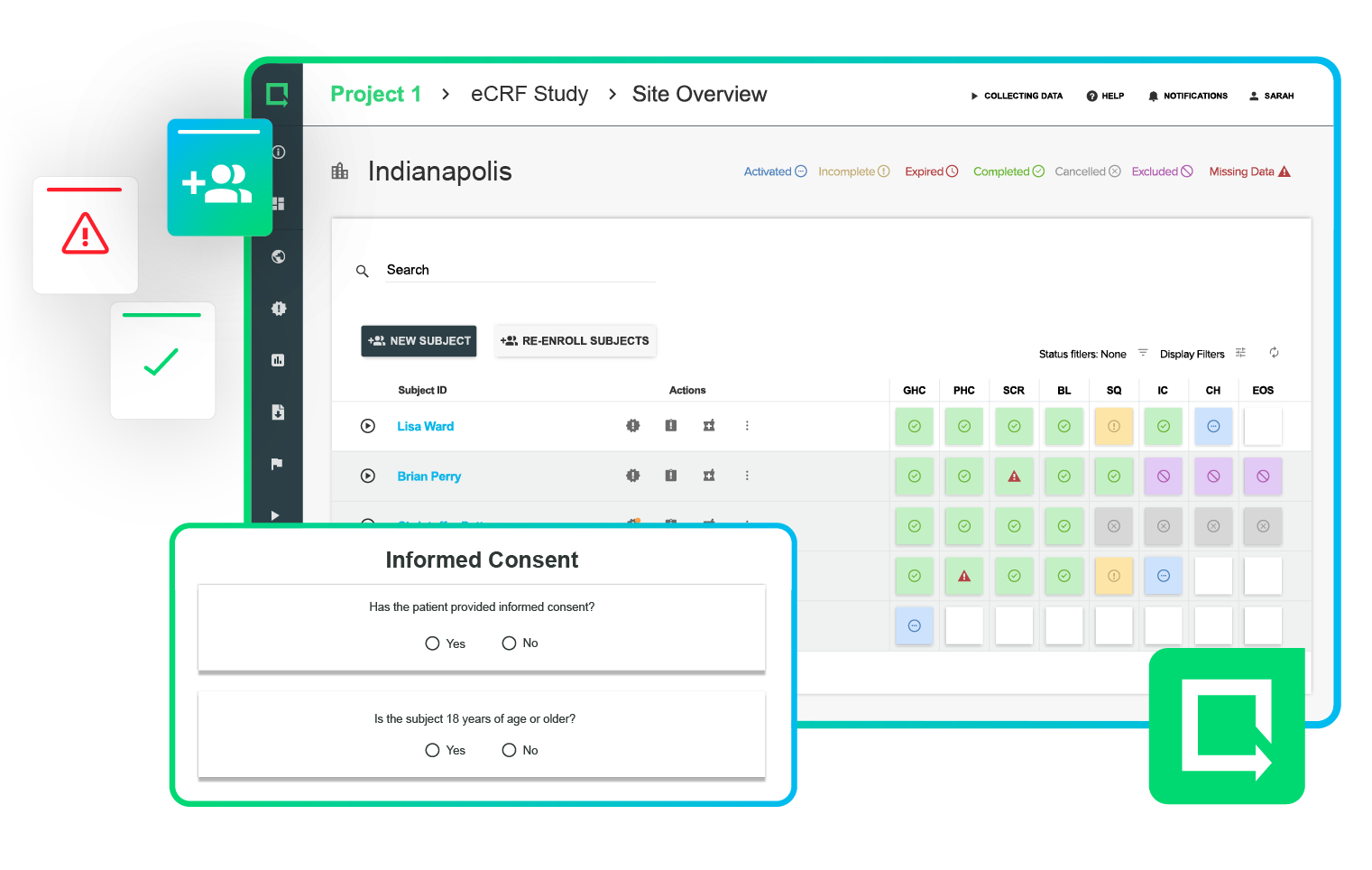
Electronically review and approve your design and development process at appropriate stages.
Part 11 compliant e-signatures, automated routing, approvals and full revision control make it easy to get the right info in front of the right stakeholders.
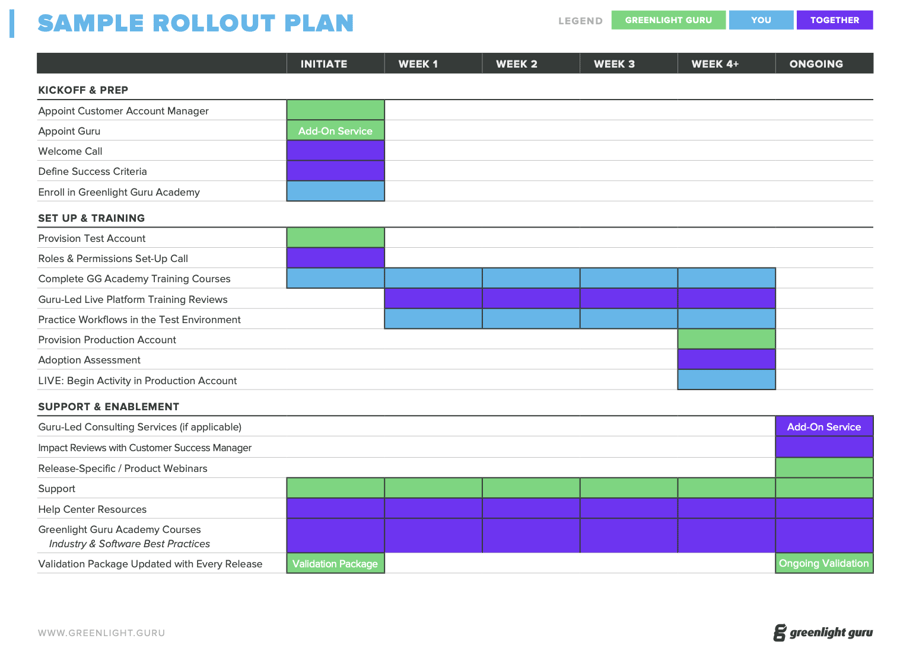
Greenlight Guru simplifies quality management and compliance. We keep our solution packages simple, too.
Our software packages are built to make it easy for you to adopt our platform. The difference amongst the packages is based on the needs of your team and business at different stages of your companies growth.
| Started in 2013 by medical device industry veterans | |
| Only True Cloud QMS with fully connected Design Controls & Risk | |
| Headquartered in Indianapolis, IN | |
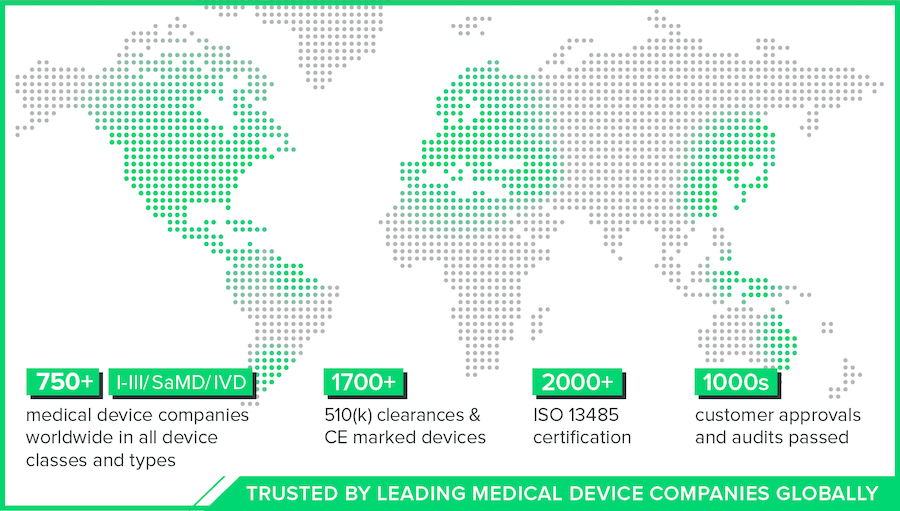
| Hundreds of customers on six continents |




A lot of times, if people don't understand quality systems, they think they just want to use paper because it presents as the cheaper option, but they fail to evaluate the total cost of ownership and impact on the business. The biggest differentiator was that it was all web based and didn't need any custom...
Having 20 years of medical device experience, I have worked with large and elaborate design controls and small and lean design controls. I have also seen the disaster resulting from the absence of appropriate design controls. Greenlight Guru is exactly big enough to ensure compliance but small enough to be easily ...
I believe the quality system I've instituted actually gives me a competitive advantage against a lot of the large companies I used to work for.
I've been thrilled with my experience working with Greenlight Guru. I am new to quality systems and compliance, and Greenlight Guru has made this transition very easy. The well-organized user interface and tracking features make the software simple and worry-free; I genuinely recommend it to anyone in the indus...
When it comes to the way they have designed the fully integrated workflows exclusively for medical device and continue to innovate with new releases, I'd call them the Tesla of medical device eQMS software.
As a design firm, our ISO 13485 certification is a competitive advantage. Greenlight Guru made getting it easy. Our designers aren't accustomed to being under a quality system, but your simple interface made it possible to adopt. It's actually working.
Using the Greenlight Guru system has enabled us to better implement our quality system across the company and accelerate our product development.
We adopted Greenlight Guru 18 months ago to build our QMS. We recently passed our ISO 13485 Stage 2 audit, due in part to the ability to demonstrate a comprehensive matrix of risk and design controls. Demonstrating our QMS using a tool like GG was fundamental in this achievement. I'll also say that the team at ...
Greenlight Guru has made the design control and risk management process extremely easy to understand and explain to people not familiar with the process and helped them to understand how all of the steps are linked. It has also made it significantly easier to get documentation reviewed and approved.
This is my first time going through a medical device product development process. I've heard about all the challenges with Design Control. With Greenlight Guru, I know what to do and when to do it. Documenting Design Controls is really easy with this software.
Your software helped us tremendously in the last few months of 2017, during which we had an important ISO 13485:2016 audit. This obviously reflected on the auditors: it was very easy to show them in what phase every project was, but also to simply show every piece of documentation we have. In the auditors conclusion...
I was actually a little nervous going into the audit, because it seemed too effortless. I've worked in QA at a Fortune 500 company with a custom solution. Your flow is better. It saves time and it doesn't break up my thought process. With Greenlight Guru I'm able to focus on quality.
We've been using Greenlight Guru for nearly 5 years now and it really simplifies quality management. It's very easy to get all of our team on the same page and effortlessly trace various efforts through the system. The Greenlight Guru team is one of the best I've ever worked with. They bend over backw...